ASBESTOS, CHRYSOTILE by XRD 9000
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download PDF About Minerals Sorted by Mineral Name
MINERALS SORTED BY NAME Here is an alphabetical list of minerals discussed on this site. More information on and photographs of these minerals in Kentucky is available in the book “Rocks and Minerals of Kentucky” (Anderson, 1994). APATITE Crystal system: hexagonal. Fracture: conchoidal. Color: red, brown, white. Hardness: 5.0. Luster: opaque or semitransparent. Specific gravity: 3.1. Apatite, also called cellophane, occurs in peridotites in eastern and western Kentucky. A microcrystalline variety of collophane found in northern Woodford County is dark reddish brown, porous, and occurs in phosphatic beds, lenses, and nodules in the Tanglewood Member of the Lexington Limestone. Some fossils in the Tanglewood Member are coated with phosphate. Beds are generally very thin, but occasionally several feet thick. The Woodford County phosphate beds were mined during the early 1900s near Wallace, Ky. BARITE Crystal system: orthorhombic. Cleavage: often in groups of platy or tabular crystals. Color: usually white, but may be light shades of blue, brown, yellow, or red. Hardness: 3.0 to 3.5. Streak: white. Luster: vitreous to pearly. Specific gravity: 4.5. Tenacity: brittle. Uses: in heavy muds in oil-well drilling, to increase brilliance in the glass-making industry, as filler for paper, cosmetics, textiles, linoleum, rubber goods, paints. Barite generally occurs in a white massive variety (often appearing earthy when weathered), although some clear to bluish, bladed barite crystals have been observed in several vein deposits in central Kentucky, and commonly occurs as a solid solution series with celestite where barium and strontium can substitute for each other. Various nodular zones have been observed in Silurian–Devonian rocks in east-central Kentucky. -

NMAM 9000: Asbestos, Chrysotile By
ASBESTOS, CHRYSOTILE by XRD 9000 MW: ~283 CAS: 12001-29-5 RTECS: CI6478500 METHOD: 9000, Issue 3 EVALUATION: FULL Issue 1: 15 May 1989 Issue 3: 20 October 2015 EPA Standard (Bulk): 1% by weight PROPERTIES: Solid, fibrous mineral; conversion to forsterite at 580 °C; attacked by acids; loses water above 300 °C SYNONYMS: Chrysotile SAMPLING MEASUREMENT BULK TECHNIQUE: X-RAY POWDER DIFFRACTION SAMPLE: 1 g to 10 g ANALYTE: Chrysotile SHIPMENT: Seal securely to prevent escape of asbestos PREPARATION: Grind under liquid nitrogen; wet-sieve SAMPLE through 10 µm sieve STABILITY: Indefinitely DEPOSIT: 5 mg dust on 0.45 µm silver membrane BLANKS: None required filter ACCURACY XRD: Copper target X-ray tube; optimize for intensity; 1° slit; integrated intensity with RANGE STUDIED: 1% to 100% in talc [1] background subtraction BIAS: Negligible if standards and samples are CALIBRATION: Suspensions of asbestos in 2-propanol matched in particle size [1] RANGE: 1% to 100% asbestos OVERALL PRECISION ( ): Unknown; depends on matrix and ESTIMATED LOD: 0.2% asbestos in talc and calcite; 0.4% concentration asbestos in heavy X-ray absorbers such as ferric oxide ACCURACY: ±14% to ±25% PRECISION ( ): 0.07 (5% to 100% asbestos); 0.10 (@ 3% asbestos); 0.125 (@ 1% asbestos) APPLICABILITY: Analysis of percent chrysotile asbestos in bulk samples. INTERFERENCES: Antigorite (massive serpentine), chlorite, kaolinite, bementite, and brushite interfere. X-ray fluorescence and absorption is a problem with some elements; fluorescence can be circumvented with a diffracted beam monochromator, and absorption is corrected for in this method. OTHER METHODS: This is NIOSH method P&CAM 309 [2] applied to bulk samples only, since the sensitivity is not adequate for personal air samples. -

State County Historic Site Name As Reported Development Latitude
asbestos_sites.xls. Summary of information of reported natural occurrences of asbestos found in geologic references examined by the authors. Dataset is part of: Van Gosen, B.S., and Clinkenbeard, J.P., 2011, Reported historic asbestos mines, historic asbestos prospects, and other natural occurrences of asbestos in California: U.S. Geological Survey Open-File Report 2011-1188, available at http://pubs.usgs.gov/of/2011/1188/. Data fields: State, ―CA‖ indicates that the site occurs in California. County, Name of the county in which the site is located. Historic site name as reported, The name of the former asbestos mine, former asbestos prospect, or reported occurrence, matching the nomenclature used in the source literature. Development, This field indicates whether the asbestos site is a former asbestos mine, former prospect, or an occurrence. "Past producer" indicates that the deposit was mined and produced asbestos ore for commercial uses sometime in the past. "Past prospect" indicates that the asbestos deposit was once prospected (evaluated) for possible commercial use, typically by trenching and (or) drilling, but the deposit was not further developed. "Occurrence" indicates that asbestos was reported at this site. The occurrence category includes (1) sites where asbestos-bearing rock is described in a geologic map or report and (2) asbestos noted as an accessory mineral or vein deposit within another type of mineral deposit. Latitude, The latitude of the site's location in decimal degrees, measured using the North American Datum of -

Iron.Rich Amesite from the Lake Asbestos Mine. Black
Canodian Mineralogist Yol.22, pp. 43742 (1984) IRON.RICHAMESITE FROM THE LAKE ASBESTOS MINE. BLACKLAKE. OUEBEC MEHMET YEYZT TANER,* AND ROGER LAURENT DAporternentde Gdologie,Universitd Loval, Qudbec,Qudbec GIK 7P4 ABSTRACT o 90.02(1l)', P W.42(12)',1 89.96(8)'.A notreconnais- sance,c'est la premibrefois qu'on ddcritune am6site riche Iron-rich amesite is found in a metasomatically altered enfer. Elles'ct form€ependant l'altdration hydrothermale granite sheet20 to 40 cm thick emplacedin serpentinite of du granitedans la serpentinite,dans les m€mes conditions the Thetford Mi[es ophiolite complex at the Lake Asbestos debasses pression et temperaturequi ont prdsid6d la for- mine (z16o01'N,11"22' W) ntheQuebec Appalachians.The mation de la rodingite dansle granite et de I'amiante- amesiteis associatedsdth 4lodingife 6semblage(grossu- chrysotiledans la serpentinite. lar + calcite t diopside t clinozoisite) that has replaced the primary minerals of the granite. The Quebec amesite Mots-clds:am6site, rodingite, granite, complexeophio- occurs as subhedral grains 2@ to 6@ pm.in diameter that litique, Thetford Mines, Qu6bec. have a tabular habit. It is optically positive with a small 2V, a 1.612,1 1.630,(t -'o = 0.018).Its structuralfor- INTRoDUc"iloN mula, calculated from electron-microprobe data, is: (Mg1.1Fe6.eA1s.e)(Alo.esil.df Os(OH)r.2. X-ray powder- Amesite is a raxehydrated aluminosilicate of mag- diffraction yield data dvalues that are systematicallygreater nesium in which some ferrous iron usually is found than those of amesitefrom Chester, Massachusetts,prob- replacingmapesium. The extent of this replacement ably becauseof the partial replacement of Mg by Fe. -

A108-316 (10/10/16)
American Industrial Hygiene Association Bulk Asbestos Proficiency Analytical Testing Program Results of Round A108-316 10/10/2016 John Herrock Laboratory ID Number Total Penalty Points 0 University of Louisiana, Monroe - Dept of 213022 Round Status P Toxicology Program Status P 700 University Ave. Monroe, LA 71209 UNITED STATES Lot Designation\Sample ID Numbers A) 1761 B) 2702 C) 1897 D) 4134 Analysis Results from Laboratory Number 213022 Asbestos (%) CHRY (3) ANTH(22) NONE CHRY (1) Other Fibrous Materials (%) FBGL (1) Nonfibrous Material (%) ACID (52) OTHR (55) ACID (60) OTHR (60) MICA (33) MICA (11) OTHR (38) ACID (29) Penalty Points Assessed 0 0 0 0 Analysis Results from Reference Laboratory One Asbestos (%) CHRY(5.8) ANTH (12) NONE CHRY (3.8) ACTN (0.1) Other Fibrous Materials (%) CELL (0.1) OTHR *1 (0.1) CELL (1) Nonfibrous Material (%) MICA (45) OTHR *2(87.9) OTHR *3 (35) PERL (20) CASO (49) OTHR *4 (65) OTHR *5 (20) OTHR *6 (55.2) Analysis Results from Reference Laboratory Two Asbestos (%) CHRY (2.5) ANTH (28) (0) CHRY(3.5%) TREM(trace) Other Fibrous Materials (%) FBGL (trace) Nonfibrous Material (%) OTHR *7 (60) OTHR *9 (24) OTHR *11 (80) OTHR *14 (20) OTHR *8(37.5) OTHR *10 (48) OTHR *12 (18) OTHR *15(76.5) OTHR *13 (2) Analysis Results from RTI International Asbestos (%) CHRY (4) ANTH (28) NONE CHRY (3) ACTN (Tra) Other Fibrous Materials (%) OTHR *16(Tra) POLY (Tra) CELL (1) OTHR *17(Tra) Nonfibrous Material (%) MICA (29) OTHR *18 (53) CACO (89) OTHR *22 (28) CASO (67) OTHR *19 (19) OTHR *20 (9) PERL (45) OTHR *21 (2) OTHR *23 -

40 Common Minerals and Their Uses
40 Common Minerals and Their Uses Aluminum Beryllium The most abundant metal element in Earth’s Used in the nuclear industry and to crust. Aluminum originates as an oxide called make light, very strong alloys used in the alumina. Bauxite ore is the main source aircraft industry. Beryllium salts are used of aluminum and must be imported from in fluorescent lamps, in X-ray tubes and as Jamaica, Guinea, Brazil, Guyana, etc. Used a deoxidizer in bronze metallurgy. Beryl is in transportation (automobiles), packaging, the gem stones emerald and aquamarine. It building/construction, electrical, machinery is used in computers, telecommunication and other uses. The U.S. was 100 percent products, aerospace and defense import reliant for its aluminum in 2012. applications, appliances and automotive and consumer electronics. Also used in medical Antimony equipment. The U.S. was 10 percent import A native element; antimony metal is reliant in 2012. extracted from stibnite ore and other minerals. Used as a hardening alloy for Chromite lead, especially storage batteries and cable The U.S. consumes about 6 percent of world sheaths; also used in bearing metal, type chromite ore production in various forms metal, solder, collapsible tubes and foil, sheet of imported materials, such as chromite ore, and pipes and semiconductor technology. chromite chemicals, chromium ferroalloys, Antimony is used as a flame retardant, in chromium metal and stainless steel. Used fireworks, and in antimony salts are used in as an alloy and in stainless and heat resisting the rubber, chemical and textile industries, steel products. Used in chemical and as well as medicine and glassmaking. -

Clay Minerals Soils to Engineering Technology to Cat Litter
Clay Minerals Soils to Engineering Technology to Cat Litter USC Mineralogy Geol 215a (Anderson) Clay Minerals Clay minerals likely are the most utilized minerals … not just as the soils that grow plants for foods and garment, but a great range of applications, including oil absorbants, iron casting, animal feeds, pottery, china, pharmaceuticals, drilling fluids, waste water treatment, food preparation, paint, and … yes, cat litter! Bentonite workings, WY Clay Minerals There are three main groups of clay minerals: Kaolinite - also includes dickite and nacrite; formed by the decomposition of orthoclase feldspar (e.g. in granite); kaolin is the principal constituent in china clay. Illite - also includes glauconite (a green clay sand) and are the commonest clay minerals; formed by the decomposition of some micas and feldspars; predominant in marine clays and shales. Smectites or montmorillonites - also includes bentonite and vermiculite; formed by the alteration of mafic igneous rocks rich in Ca and Mg; weak linkage by cations (e.g. Na+, Ca++) results in high swelling/shrinking potential Clay Minerals are Phyllosilicates All have layers of Si tetrahedra SEM view of clay and layers of Al, Fe, Mg octahedra, similar to gibbsite or brucite Clay Minerals The kaolinite clays are 1:1 phyllosilicates The montmorillonite and illite clays are 2:1 phyllosilicates 1:1 and 2:1 Clay Minerals Marine Clays Clays mostly form on land but are often transported to the oceans, covering vast regions. Kaolinite Al2Si2O5(OH)2 Kaolinite clays have long been used in the ceramic industry, especially in fine porcelains, because they can be easily molded, have a fine texture, and are white when fired. -

Chrysotile Asbestos
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. Environmental Health Criteria 203 CHRYSOTILE ASBESTOS First draft prepared by Dr G. Gibbs, Canada (Chapter 2), Mr B.J. Pigg, USA (Chapter 3), Professor W.J. Nicholson, USA (Chapter 4), Dr A. Morgan, UK and Professor M. Lippmann, USA (Chapter 5), Dr J.M.G. Davis, UK and Professor B.T. Mossman, USA (Chapter 6), Professor J.C. McDonald, UK, Professor P.J. Landrigan, USA and Professor W.J. Nicholson, USA (ChapterT), Professor H. Schreier, Canada (Chapter 8). Published under the joint sponsorship of the United Nations Environment Progralnme, the International Labour Organisation, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals. World Health Organization Geneva, 1998 The International Programme on chemicat safety (Ipcs), esrablished in 1980, is a joint venture of the united Nations Environment programme (uNEp), the International l-abour organisation (ILo), and the world ueatttr orginization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure rc chemicals, through international peer review processes, as a prerequisiie for the promotion of chemical safety, and to provide technical assistance -

Chrysotile Asbestos As a Cause of Mesothelioma: Application of the Hill Causation Model
Commentary Chrysotile Asbestos as a Cause of Mesothelioma: Application of the Hill Causation Model RICHARD A. LEMEN, PHD Chrysotile comprises over 95% of the asbestos used this method, researchers are asked to evaluate nine today. Some have contended that the majority of areas of consideration: strength of association, tempo- asbestos-related diseases have resulted from exposures rality, biologic gradient, consistency, specificity, bio- to the amphiboles. In fact, chrysotile is being touted as logic plausibility, coherence, experimental evidence, the form of asbestos which can be used safely. Causa- and analogy. None of these considerations, in and of tion is a controversial issue for the epidemiologist. How itself, is determinative for establishing a causal rela- much proof is needed before causation can be estab- tionship. As Hill himself noted, “[n]one of my nine lished? This paper examines one proposed model for establishing causation as presented by Sir Austin Brad- view points can bring indisputable evidence for or ford Hill in 1965. Many policymakers have relied upon against the cause and effect hypothesis, and none can this model in forming public health policy as well as be required as a sine qua non.” In the same vein, it is deciding litigation issues. Chrysotile asbestos meets not necessary for all nine considerations to be met Hill’s nine proposed criteria, establishing chrysotile before causation is established. Instead, Hill empha- asbestos as a cause of mesothelioma. Key words: sized that the responsibility for making causal judg- asbestos; chrysotile; amphiboles; causation; mesothe- ments rested with a scientific evaluation of the totality lioma; Hill model. of the data. -

A RARE-ALKALI BIOTITE from KINGS MOUNTAIN, NORTH CAROLINA1 Fnanr L
A RARE-ALKALI BIOTITE FROM KINGS MOUNTAIN, NORTH CAROLINA1 FnaNr L. Hnss2 arqn Ror-r.rx E. SrrvrNs3 Severalyears ago, after Judge Harry E. Way of Custer, South Dakota, had spectroscopically detected the rare-alkali metals in a deep-brown mica from a pegmatite containing pollucite and lithium minerals, in Tin Mountain, 7 miles west of Custer, another brown mica was collected, which had developed notably in mica schist at its contact with a similar mass of pegmatite about one half mile east of Tin Mountai". J. J. Fahey of the United States GeolgoicalSurvey analyzed the mica, and it proved to contain the rare-alkali metalsaand to be considerably difierent from any mica theretofore described. Although the cesium-bearing minerals before known (pollucite, lepidolite, and beryl) had come from the zone of highest temperature in the pegmatite, the brown mica was from the zone of lowest temperature. The occurrence naturally suggestedthat where dark mica was found developed at the border of a pegmatite, especially one carrying lithium minerals, it should be examined for the rare-alkali metals. As had been found by Judge Way, spectroscopictests on the biotite from Tin Moun- tain gave strong lithium and rubidium lines, and faint cesium lines. Lithium lines were shown in a biotite from the border of the Morefield pegmatite, a mile south of Winterham, Virginia, but rubidium and cesium w'erenot detected. $imilarly placed dark micas from Newry and Hodgeon HiII, near Buckfield, Maine, gave negative results. They should be retested. Tests by Dr. Charles E. White on a shiny dark mica from the Chestnut FIat pegmatite near Spruce Pine, North Carolina, gave strong lithium and weaker cesium lines. -
Mica Data Sheet
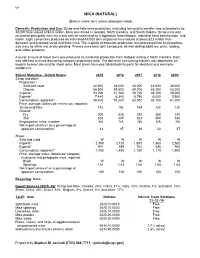
108 MICA (NATURAL) (Data in metric tons unless otherwise noted) Domestic Production and Use: Scrap and flake mica production, excluding low-quality sericite, was estimated to be 38,000 tons valued at $4.6 million. Mica was mined in Georgia, North Carolina, and South Dakota. Scrap mica was recovered principally from mica and sericite schist and as a byproduct from feldspar, industrial sand beneficiation, and kaolin. Eight companies produced an estimated 63,000 tons of ground mica valued at about $22 million from domestic and imported scrap and flake mica. The majority of domestic production was processed into small-particle- size mica by either wet or dry grinding. Primary uses were joint compound, oil-well-drilling additives, paint, roofing, and rubber products. A minor amount of sheet mica was produced as incidental production from feldspar mining in North Carolina. Data was withheld to avoid disclosing company proprietary data. The domestic consuming industry was dependent on imports to meet demand for sheet mica. Most sheet mica was fabricated into parts for electrical and electronic equipment. Salient Statistics—United States: 2015 2016 2017 2018 2019e Scrap and flake: Production:1 Sold and used 32,600 28,000 40,000 44,000 38,000 Ground 65,800 59,500 69,700 65,300 63,000 Imports2 33,200 31,500 29,700 28,100 29,000 Exports3 7,440 6,340 6,790 6,000 5,900 Consumption, apparent4 58,400 53,200 62,900 66,100 61,000 Price, average, dollars per metric ton, reported: Scrap and flake 142 152 165 122 120 Ground: Dry 305 320 292 308 310 Wet 423 435 424 454 480 Employment, mine, number NA NA NA NA NA Net import reliance5 as a percentage of apparent consumption 44 47 36 33 37 Sheet: Sold and used W W W W W Imports6 2,390 2,120 1,850 1,860 2,500 Exports7 911 689 704 686 950 Consumption, apparent5 1,480 1,430 1,150 1,170 1,600 Price, average value, dollars per kilogram, muscovite and phlogopite mica, reported: Block W W W W W Splittings 1.61 1.61 1.66 1.65 1.65 Net import reliance5 as a percentage of apparent consumption 100 100 100 100 100 Recycling: None. -

List of Abbreviations
List of Abbreviations Ab albite Cbz chabazite Fa fayalite Acm acmite Cc chalcocite Fac ferroactinolite Act actinolite Ccl chrysocolla Fcp ferrocarpholite Adr andradite Ccn cancrinite Fed ferroedenite Agt aegirine-augite Ccp chalcopyrite Flt fluorite Ak akermanite Cel celadonite Fo forsterite Alm almandine Cen clinoenstatite Fpa ferropargasite Aln allanite Cfs clinoferrosilite Fs ferrosilite ( ortho) Als aluminosilicate Chl chlorite Fst fassite Am amphibole Chn chondrodite Fts ferrotscher- An anorthite Chr chromite makite And andalusite Chu clinohumite Gbs gibbsite Anh anhydrite Cld chloritoid Ged gedrite Ank ankerite Cls celestite Gh gehlenite Anl analcite Cp carpholite Gln glaucophane Ann annite Cpx Ca clinopyroxene Glt glauconite Ant anatase Crd cordierite Gn galena Ap apatite ern carnegieite Gp gypsum Apo apophyllite Crn corundum Gr graphite Apy arsenopyrite Crs cristroballite Grs grossular Arf arfvedsonite Cs coesite Grt garnet Arg aragonite Cst cassiterite Gru grunerite Atg antigorite Ctl chrysotile Gt goethite Ath anthophyllite Cum cummingtonite Hbl hornblende Aug augite Cv covellite He hercynite Ax axinite Czo clinozoisite Hd hedenbergite Bhm boehmite Dg diginite Hem hematite Bn bornite Di diopside Hl halite Brc brucite Dia diamond Hs hastingsite Brk brookite Dol dolomite Hu humite Brl beryl Drv dravite Hul heulandite Brt barite Dsp diaspore Hyn haiiyne Bst bustamite Eck eckermannite Ill illite Bt biotite Ed edenite Ilm ilmenite Cal calcite Elb elbaite Jd jadeite Cam Ca clinoamphi- En enstatite ( ortho) Jh johannsenite bole Ep epidote