Utility of Testing for Apraxia and Associated Features in Dementia Samrah Ahmed,1 Ian Baker,2 Sian Thompson,3 Masud Husain,1,4 Christopher R Butler1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dyslexia and Dyscalculia Are the Same Thing
Dyscalculia and Dyslexia Two different issues, or part of the same problem? By Tony Attwood C.Ed., B.A., M.Phil. Chairman of The Dyscalculia Group, First and Best in Education Ltd. Dyscalculia is noted as an unexpected difficulty that some people have in dealing with mathematical problems. At its simplest we may note a child whose age and intellect suggests that he or she will be able to undertake a certain range of skills, but who in effect is unable to handle maths problems that we would expect to be well within his or her grasp. As a general introduction to the topic we may note that according to the American Journal of Paediatrics in 2001 dyscalculic children have “Normal or advanced language and other skills, often good visual memory for the printed word, (and) poor mental maths ability often with difficulty in the use of money, such as balancing a chequebook, making change and tipping. Often there is a fear of money and its transactions and difficulty with maths processes (e.g. addition, subtraction, multiplication) and concepts (e.g. sequencing of numbers). There is sometimes poor retention and retrieval of concepts, or an inability to maintain a consistency in grasping maths rules, poor sense of direction, easy disorientation, as well as difficulty with reading maps, telling the time and grappling with mechanical processes, difficulty with abstract concepts of time and direction, schedules, keeping track of time and the sequence of past and future events.” These children make, “Common mistakes in working with numbers including number reversals and omissions,” (and) “May have difficulty learning musical concepts, following directions in sports that demand sequencing or rules, and keeping track of scores and players during games such as cards and board games. -

Developmental Coordination Disorder and Dysgraphia
Developmental coordination disorder and dysgraphia: signs and symptoms, diagnosis, and rehabilitation Maëlle Biotteau, Jérémy Danna, Éloïse Baudou, Frédéric Puyjarinet, Jean-Luc Velay, Jean-Michel Albaret, Yves Chaix To cite this version: Maëlle Biotteau, Jérémy Danna, Éloïse Baudou, Frédéric Puyjarinet, Jean-Luc Velay, et al.. De- velopmental coordination disorder and dysgraphia: signs and symptoms, diagnosis, and rehabil- itation. Neuropsychiatric Disease and Treatment, Dove Medical Press, 2019, 15, pp.1873-1885. 10.2147/NDT.S120514. hal-02177153 HAL Id: hal-02177153 https://hal.archives-ouvertes.fr/hal-02177153 Submitted on 8 Jul 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Neuropsychiatric Disease and Treatment Dovepress open access to scientific and medical research Open Access Full Text Article REVIEW Developmental coordination disorder and dysgraphia: signs and symptoms, diagnosis, and rehabilitation This article was published in the following Dove Press journal: Neuropsychiatric Disease and Treatment Maëlle Biotteau 1 Abstract: Developmental coordination disorder (DCD) is a common and well-recognized Jérémy Danna 2 neurodevelopmental disorder affecting approximately 5 in every 100 individuals worldwide. It Éloïse Baudou 3 has long been included in standard national and international classifications of disorders (especially Frédéric Puyjarinet 4 the Diagnostic and Statistical Manual of Mental Disorders). -

The Basics About the 3 D's: Learning Disabilities
THE BASICS ABOUT THE 3 D’S: LEARNING DISABILITIES Cathy Johnson, M.A., C.C.C, S.L.T Licensed Speech/Language Pathologist Structured Literacy Teacher Speech, Language and Learning Center Johnson Academy of Therapeutic Learning Disclosure Neither I nor any member of my immediate family has a financial relationship or interest (currently or within the past 12 months) with any proprietary entity producing health care goods or services consumed by, or used on, patients related to the content of this CME activity. I do not intend to discuss an unapproved/investigative use of a commercial product/device. Agenda ● Three types of learning disabilities: Dyslexia, Dysgraphia, and Dyscalculia ● Definitions ● Signs and symptoms ● Frequently co‐occurring disorders to be aware of: ADHD (30% of those with dyslexia have coexisting AD/HD) &/or APD Learning Disabilities • Problems with age appropriate reading, spelling, and/or writing • A learning disability is not about how smart a person is but more about how they process sounds and language • Most people diagnosed with learning disabilities have average to superior intelligence Definition of Dyslexia ● Dyslexia is no longer diagnosed with regard to an IQ discrepancy. ● We have known this from research that came out in the early 1990s (e.g., Siegel 1992). ● This was officially changed in the DSM‐5 (2013). Definition of Dyslexia- IDA definition average to above average intellectual ability with an unexpected difficulty in reading 1. Dyslexia is a language‐based learning disability. 2. Dyslexia is hereditary and lifelong. 3. Dyslexia is more common than many people think. 4. Before school starts, dyslexia may not be obvious. -
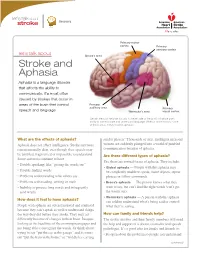
Stroke and Aphasia Aphasia Is a Language Disorder That Affects the Ability to Communicate
Recovery Primary motor cortex Primary sensory cortex let’s talk about Broca’s area Stroke and Aphasia Aphasia is a language disorder that affects the ability to communicate. It’s most often caused by strokes that occur in areas of the brain that control Primary auditory area Primary speech and language. Wernicke’s area visual cortex Certain areas of the brain (usually in the left side of the brain) influence one’s ability to communicate and understand language. When a stroke occurs in one of these areas, it may result in aphasia. What are the effects of aphasia? sender plissen.” Thousands of alert, intelligent men and Aphasia does not affect intelligence. Stroke survivors women are suddenly plunged into a world of jumbled remain mentally alert, even though their speech may communication because of aphasia. be jumbled, fragmented or impossible to understand. Are there different types of aphasia? Some survivors continue to have: Yes, there are several forms of aphasia. They include: • Trouble speaking, like “getting the words out” • Global aphasia — People with this aphasia may • Trouble finding words be completely unable to speak, name objects, repeat • Problems understanding what others say phrases or follow commands. • Problems with reading, writing or math • Broca’s aphasia — The person knows what they • Inability to process long words and infrequently want to say, but can’t find the right words (can’t get used words the words out). • Wernicke’s aphasia — A person with this aphasia How does it feel to have aphasia? can seldom understand what’s being said or control People with aphasia are often frustrated and confused what they’re saying. -

Abadie's Sign Abadie's Sign Is the Absence Or Diminution of Pain Sensation When Exerting Deep Pressure on the Achilles Tendo
A.qxd 9/29/05 04:02 PM Page 1 A Abadie’s Sign Abadie’s sign is the absence or diminution of pain sensation when exerting deep pressure on the Achilles tendon by squeezing. This is a frequent finding in the tabes dorsalis variant of neurosyphilis (i.e., with dorsal column disease). Cross References Argyll Robertson pupil Abdominal Paradox - see PARADOXICAL BREATHING Abdominal Reflexes Both superficial and deep abdominal reflexes are described, of which the superficial (cutaneous) reflexes are the more commonly tested in clinical practice. A wooden stick or pin is used to scratch the abdomi- nal wall, from the flank to the midline, parallel to the line of the der- matomal strips, in upper (supraumbilical), middle (umbilical), and lower (infraumbilical) areas. The maneuver is best performed at the end of expiration when the abdominal muscles are relaxed, since the reflexes may be lost with muscle tensing; to avoid this, patients should lie supine with their arms by their sides. Superficial abdominal reflexes are lost in a number of circum- stances: normal old age obesity after abdominal surgery after multiple pregnancies in acute abdominal disorders (Rosenbach’s sign). However, absence of all superficial abdominal reflexes may be of localizing value for corticospinal pathway damage (upper motor neu- rone lesions) above T6. Lesions at or below T10 lead to selective loss of the lower reflexes with the upper and middle reflexes intact, in which case Beevor’s sign may also be present. All abdominal reflexes are preserved with lesions below T12. Abdominal reflexes are said to be lost early in multiple sclerosis, but late in motor neurone disease, an observation of possible clinical use, particularly when differentiating the primary lateral sclerosis vari- ant of motor neurone disease from multiple sclerosis. -

What This Means for You As a Special Educator/Service Provider
Anne Arundel County – Department of Special Education Technical Assistant Bulletin (revised July 2017) Specific Learning Disability; Including Dyslexia; Dysgraphia; Dyscalculia September 2019 Overall Emphasis and Purpose: Specific Learning Disability (SLD) is one of the 13 categories of disability recognized by the IDEA. SLD is the only disability category for which the IDEA establishes special evaluation procedures, in addition to the general procedures that are used for all students with disabilities. The definition of a SLD is “a disorder in one or more of the basic psychological processes involved in understanding, or in using language spoken or written, that may manifest itself in the imperfect ability to listen, think, speak, read, write, spell, or do mathematical calculations.” During the 2017 legislative session, a bill was passed authorizing schools to identify dyslexia, dysgraphia, or dyscalculia, as subcategories under the overarching SLD classification. What this means for you as a Special What the Special Educator/Service Educator/Service Provider: Provider needs to do if not already doing: • The identification of a SLD requires that the student • Ensure that school staff are supported in implementing exhibit a pattern of strengths and weaknesses in research and/or evidence-based intervention for any performance, achievement, or both, relative to age, student who is struggling. Progress monitoring is a key State approved grade level standards, or intellectual component of determining the effectiveness of the development, as a result of a comprehensive intervention prior to considering the possibility of a evaluation. disability under the IDEA. • A student identified with a specific learning disability, • The IEP team must consider exclusionary factors, such with or without dyslexia, dysgraphia, or dyscalculia, as a lack of appropriate education, intellectual must also exhibit educational impact and require disability, visual, hearing, or motor impairment, specialized instruction. -
• Classifications of Aphasia Expressive Vs. Receptive Fluent Vs
12/7/2018 APHASIA Aphasia is an acquired communication disorder that impairs a person’s ability to process LANGUAGE, but DOES NOT AFFECT intelligence. Aphasia impairs the ability to speak and understand others. -National Aphasia Association LANGUAGE Language is a system of communication that uses symbolism. K L U $ + M – Phonemes: perceptually distinct unit of sounds Words: sounds combined & given meaning Sentences: combination of syntax (rules) and semantics (meaning). • CLASSIFICATIONS OF APHASIA EXPRESSIVE VS. RECEPTIVE FLUENT VS. NON- FLUENT 1 12/7/2018 -NATIONAL APHASIA ASSOCIATION -COURTESY OF MY-MS.ORG MCA DISTRIBUTION -SLIDESHARE.NET 2 12/7/2018 BROCA’S APHASIA * short utterances * limited vocabulary * halting, effortful speech *mild comprehension deficits Lesion * Inferior frontal gyrus Choose Sentence Speech Coordinate Speak Idea Words Structure Sounds Articulate Pragmatics Muscles Fluently (Semantics) (Syntax) (Phonology) SAMPLE OF BROCA’S THERAPY FROM TACTUS THERAPY 3 12/7/2018 WERNICKE’S APHASIA • Comprehension is poor (auditory & reading) • Fluent, intact prosody • Logorrhea, press of speech • Neologisms, Paraphasias • Lack of awareness Lesion Temporo-Parietal, Posterior section of the superior temporal gyrus near the auditory cortex Auditory Preparation Attach Input Perception Recognition Phonological For Meaning Analysis Output WERNICKE’S APHASIA FROM TACTUS THERAPY 4 12/7/2018 GLOBAL APHASIA * severe language deficit * responds to personally relevant language * responds to non-verbal cues * some automatic speech Lesion -

Developmental Dyscalculia, Gender, and the Brain
510 ArchivesofDiseasein Childhood 1993; 68: 510-512 CURRENT TOPIC Arch Dis Child: first published as 10.1136/adc.68.4.510 on 1 April 1993. Downloaded from Developmental dyscalculia, gender, and the brain Varda Gross-Tsur, Orly Manor, Ruth S Shalev Background Aldenkamp reported that children in the 12-18 Developmental dyscalculia is a primary cognitive year age group had the most difficulties with disorder of childhood affecting the ability of an mathematics. 14 Seidenberg et al studied the otherwise intelligent and healthy child to learn academic achievement of 122 children ages 7-15 arithmetic.' Preliminary evidence indicates that years and found that this group was making less developmental dyscalculia is seen in 5-6% of academic progress than expected for their age normal children23 and is as prevalent as develop- and level of intelligence quotient (IQ); academic mental dyslexia or the attention deficit hyper- deficiencies were greatest in the area of arith- activity disorder.' One of the classifications of metic followed by spelling, reading comprehen- developmental dyscalculia subdivides dys- sion, and word recognition skills.'5 It has been calculia to: (1) alexia and agraphia for numbers, postulated that the 'speed factor type' oflearning (2) spatial dyscalculia, (3) anarithmetia (impair- disability in epileptic children was found to be ment of calculation per se), (4) attentional- related to underachievement in arithmetic.8 sequential dyscalculia, and (5) mixed type.4 Early age of seizure onset, age of the child, Rourke and Finlayson -

Do Children with Developmental Dyscalculia Havean Orderprocessing Deficit?
Do children with Developmental Dyscalculia have an order processing deficit? Kinga Morsanyi, Bianca van Bers and Teresa McCormack, Queen’s University Belfast 61 60 59 58 57 56 62 41 40 39 38 37 42 25 24 23 22 55 63 26 13 12 11 36 43 14 5 4 21 54 64 27 6 1 10 35 44 15 2 3 20 53 65 28 7 8 9 34 45 16 17 18 19 52 66 29 30 31 32 33 46 47 48 49 50 51 Contents Research highlights 2 Executive summary 5 The need for this research 7 Summary of aims 8 Methods 9 Key findings 14 Summary of key findings and recommendations 17 References 21 Acknowledgements 23 Appendix: Infographics 24 1 Research highlights Study 1: The prevalence of specific learning disorder in mathematics (SLDM or dyscalculia)1 This was the first prevalence study of SLDM since the publication of the new DSM-5 (Diagnostic and Statistical Manual of Mental Disorders) diagnostic criteria in 2013. We considered data from 2,421 children (their level of intelligence and educational achievement in mathematics and English were recorded over several school years). We investigated the effects of gender, socio-economic status, special educational needs (other than issues related to mathematics), and whether the child spoke English as their first language on mathematics achievement. 5.7% of the sample was identified as having SLDM. Compared to earlier (DSM-IV) diagnostic criteria, the prevalence of SLDM was almost 6 times higher. A child was more than a 100 times more likely to receive a diagnosis of dyslexia than SLDM, although prevalence rates are expected to be similar. -

WORKING MEMORY & LEARNING DISABILITIES Working Memory Is
3/26/14 Working Memory is the FOUNDATION of Learning WORKING MEMORY & LEARNING DISABILITIES Tracy Packiam Alloway, PhD WM & Learning DisabiliKes DYSLEXIA (READING) • WHAT is the Core Deficit? • WHY is Working Memory is involved? • HOW to support Working Memory? 1 3/26/14 DYSLEXIA (READING) DYSLEXIA (READING) GENERAL STRATEGIES GENERAL STRATEGIES • Reduce working memory processing in • Keep track of their place in complex activities acKviKes – History Kmelines Rulers: reading & math problems – 45 + 98 45 +98 DYSLEXIA (READING) MATH (DYSCALCULIA) SPECIFIC STRATEGIES • Shorten acKviKes to reduce WM load – Sam worked with only 5 flowers – Repeated instructions just for him www.tracyalloway.com 2 3/26/14 Math (Dyscalculia) Math (Dyscalculia) GENERAL STRATEGIES GENERAL STRATEGIES • Use visual representaon to support working memory • Use visual representaon to support working memory • Algebra: negative exponents • Algebra: negative exponents www.tracyalloway.com Math (Dyscalculia) DCD (Motor) • Gross motor skills (large movements): SPECIFIC STRATEGIES – Poor balance: Riding a bicycle • Model the use of memory aids – Poor hand-eye co-ordination: Catching a ball & batting – Number lines • Fine motor skills (small movements): – Lack of manual dexterity: using cutlery, craft work, 1, 2, __, 4, __ playing musical instruments – Poor manipulative skills: Typing, handwriting and drawing, fastening clothes & tying shoelaces www.tracyalloway.com Alloway (2006) Working Memory & Neurodevelopmental Disorders. Psy Press 3 3/26/14 DCD (MOTOR) DCD (Motor) • Motor skills or Working Memory = Learning difficulties? • Two groups: – High Visual-Spatial Memory – Low Visual-Spatial Memory • Motor skills: Both groups will have low learning scores • Working Memory: Low VS Memory group will have lower learning scores • Low Visual-Spatial Memory group performed worse in Reading & Math – Even after accounting for IQ Alloway (2007) J. -

Number Reading in Pure Alexiaâ
Neuropsychologia 49 (2011) 2283–2298 Contents lists available at ScienceDirect Neuropsychologia jo urnal homepage: www.elsevier.com/locate/neuropsychologia Reviews and perspectives Number reading in pure alexia—A review a,∗ b Randi Starrfelt , Marlene Behrmann a Center for Visual Cognition, Department of Psychology, Copenhagen University, O. Farimagsgade 2A, DK-1353 Copenhagen K, Denmark b Department of Psychology, Carnegie Mellon University, Pittsburgh, PA, USA a r t i c l e i n f o a b s t r a c t Article history: It is commonly assumed that number reading can be intact in patients with pure alexia, and that this Received 25 October 2010 dissociation between letter/word recognition and number reading strongly constrains theories of visual Received in revised form 31 March 2011 word processing. A truly selective deficit in letter/word processing would strongly support the hypothesis Accepted 22 April 2011 that there is a specialized system or area dedicated to the processing of written words. To date, however, Available online 4 May 2011 there has not been a systematic review of studies investigating number reading in pure alexia and so the status of this assumed dissociation is unclear. We review the literature on pure alexia from 1892 to Keywords: 2010, and find no well-documented classical dissociation between intact number reading and impaired Pure alexia letter identification in a patient with pure alexia. A few studies report strong dissociations, with number Alexia without agraphia reading less impaired than letter reading, but when we apply rigorous statistical criteria to evaluate Letter-by-letter reading Visual recognition these dissociations, the difference in performance across domains is not statistically significant. -

Apraxia Neurology, Neuropsychology and Rehabilitation
Apraxia Neurology, Neuropsychology and Rehabilitation Jon Marsden Professor of Rehabilitation School of Health Professions Tool Use D. Stout, T. Chaminade / Neuropsychologia 45 (2007) 1091–1100 http://www.bradshawfoundation.com/origins/oldowan_stone_tools.php Action Steams in the Brain Apraxia Definitions, Prevalence and Impact Ideomotor Ideational Apraxia Apraxia Rehabilitation and Recovery of Apraxia Action Streams in the Brain What and How in the Visual System Posterior Parietal Cortex Dorsal stream Primary /Secondary Retina LGNd Visual Cortex Ventral stream Inferotemporal cortex Goodale et al (1996) In Hand and Brain. Visuomotor Control REACHING GRASPING PE-F2 AIP-F5 Directional Transforming visual Coding to objects intrinsic In extra-personal Object attributes into Space Motor commands Superior Longitudinal Fasciculus Rizzolatti and Luppino TOOL MANPULATION (2001), Neuron 31 889-901 VIP-F4 Bimodal receptive fields Sensorimotor Transformation RF altered by tool use in Parieto-Frontal Circuits Modification of Body Schema by Tools Visual receptive Field Somatosensory Receptive Field Inter-parietal Neurons Maravita and Iriki 204 TICS 79-86 Action Streams in the brain Bilateral Dorso-dorsal system “Grasp” (actions to visual targets) X2 action systems Vs ventral and Left lateralised Ventrodorsal dorsal stream system Interaction “Use” devoted to skilled functional Adapted from Liepmann 1920 Binkofski et al 2013 Brain and Language 127 22-229 object use What Is Apraxia Definitions, Prevalence and Impact What is Apraxia? A disorder of skilled