RD108-The Effect of Secondary Ettringite Formation on the Durability of Concrete-A Literature Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Charlesite, a New Mineral of the Ettringite Group, from Franklin, New Jersey
American Mineralogist, Volume 68, pages 1033-1037,1983 Charlesite, a new mineral of the ettringite group, from Franklin, New Jersey PBre J. DuxN Department of Mineral Sciences SmithsonianInstitution, Washington,D. C. 20560 DoNero R. Peecon Department of GeologicalSciences University of Michigan, Ann Arbor, Michigan 48109 PBrnn B. LBavBNs Departmentof Geology Universityof Delaware, Newark, Delaware l97ll eNo JonN L. Beuu Franklin Mineral Museum Franklin. New Jersey 07416 Abstract Charlesite,ideally C4(AI,Si)z(SO4)2(B(OH)4)(OH,O)r2.26H2Ois a member of the ettrin- gite group from Franklin, New Jersey, and is the Al analogueof sturmanite. Chemical analysisyielded CaO27.3, Al2O3 5.1, SiO2 3.1, SO3 12.8,B2o33.2, H2O 48.6, sum : 100.1 percent.-Charlesiteis hexagonal,probable spacegroup P3lc, with a = ll.16(l), c = 21.21(2)4. The strongest lines in the X-ray powder difraction pattern (d, IlIo, hkl) are: 9.70,100, 100;5.58, 80, 110;3.855,80, ll4;2.749,70,304;2.538,70,126;2.193,70,2261 404. Charlesite occurs as simple hexagonal crystals tabular on {0001} and has a perfect {10T0}cleavage. The densityis 1.77glcm3 (obs.) and 1.79glcms (calc.). Optically, charlesite is uniaxial( -) with a : | .492(3)and e : 1.475(3).It occurswith clinohedrite,ganophyllite, xonotlite, prehnite, roeblingite and other minerals in severalparageneses at Franklin, New Jersey. Charlesite is named in honor of the late Professor Charles Palache. Introduction were approved, prior to publication, by the Commission Minerals and Mineral Names. I. M. A. The An ettringite-like mineral was first described from on New specimenwas divided into three portions. -

General Index
CAL – CAL GENERAL INDEX CACOXENITE United States Prospect quarry (rhombs to 3 cm) 25:189– Not verified from pegmatites; most id as strunzite Arizona 190p 4:119, 4:121 Campbell shaft, Bisbee 24:428n Unanderra quarry 19:393c Australia California Willy Wally Gully (spherulitic) 19:401 Queensland Golden Rule mine, Tuolumne County 18:63 Queensland Mt. Isa mine 19:479 Stanislaus mine, Calaveras County 13:396h Mt. Isa mine (some scepter) 19:479 South Australia Colorado South Australia Moonta mines 19:(412) Cresson mine, Teller County (1 cm crystals; Beltana mine: smithsonite after 22:454p; Brazil some poss. melonite after) 16:234–236d,c white rhombs to 1 cm 22:452 Minas Gerais Cripple Creek, Teller County 13:395–396p,d, Wallaroo mines 19:413 Conselheiro Pena (id as acicular beraunite) 13:399 Tasmania 24:385n San Juan Mountains 10:358n Renison mine 19:384 Ireland Oregon Victoria Ft. Lismeenagh, Shenagolden, County Limer- Last Chance mine, Baker County 13:398n Flinders area 19:456 ick 20:396 Wisconsin Hunter River valley, north of Sydney (“glen- Spain Rib Mountain, Marathon County (5 mm laths donite,” poss. after ikaite) 19:368p,h Horcajo mines, Ciudad Real (rosettes; crystals in quartz) 12:95 Jindevick quarry, Warregul (oriented on cal- to 1 cm) 25:22p, 25:25 CALCIO-ANCYLITE-(Ce), -(Nd) cite) 19:199, 19:200p Kennon Head, Phillip Island 19:456 Sweden Canada Phelans Bluff, Phillip Island 19:456 Leveäniemi iron mine, Norrbotten 20:345p, Québec 20:346, 22:(48) Phillip Island 19:456 Mt. St-Hilaire (calcio-ancylite-(Ce)) 21:295– Austria United States -

The Lead Silicates from Franklin, New Jersey: Occurrence and Composition
MINERALOGICAL MAGAZINE, DECEMBER 1985, VOL. 49, PP. 7217 The lead silicates from Franklin, New Jersey: occurrence and composition PETE J. DUNN Department of Mineral Sciences, Smithsonian Institution, Washington, DC 20560, USA ABSTRACT. The lead silicate minerals from Franklin, History and occurrence New Jersey, occurred in two separate assemblages. One of these is characterized by esperite associated with hardy- Inasmuch as the Franklin Mine was mined-out stonite and occasionallarsenite. The second assemblage and flooded in 1954, examination of the geologic can be considered as two parts: one consists of margaro- relations of the occurrences of the lead silicates was sanite, barysilite, nasonite, and ganomalite; the other not possible. Recourse was made to mine maps and contains roeblingite and hancockite, together with a interviews with former employees ofthe New Jersey number of highly hydrated phases. Chemical analyses Zinc Company who had examined these occur- indicate that these species conform to their theoretical rences, most notably John L. Baum, retired resident compositions. There are no simple lead siJicates at geologist for the Franklin Mine. Franklin; al1 are compound silicates of Pb with Mn, Zn, and Ca. KEYWORDS: lead silicates, barysilite, esperite, ganomal- Table l. The lead silicate minerals found at ite, hancockite, larsenite, margarosanite, nasonite, roe- Franltlin, New Jersey blingite, Franklin, New Jersey, USA. BARYSILITE PbSMn(Si207)3 ATthe end of the 19th century a remarkable suite of ESPERITE ca3PbZn4(Si04)4 uncommon minerals was found on the Parker GANOMALITE Pb9CaSMnSi9ÜJ3 dump, near the Parker shaft of the Franklin Mine, (OH) in Franklin, Sussex County, New Jersey. This suite HANCOCKITE PbCa(A1,Fe3+)3(Si04)3 inc\uded a number of unknown minerals which LARSENITE PbZnSi04 were subsequently described as new species. -

Shin-Skinner January 2018 Edition
Page 1 The Shin-Skinner News Vol 57, No 1; January 2018 Che-Hanna Rock & Mineral Club, Inc. P.O. Box 142, Sayre PA 18840-0142 PURPOSE: The club was organized in 1962 in Sayre, PA OFFICERS to assemble for the purpose of studying and collecting rock, President: Bob McGuire [email protected] mineral, fossil, and shell specimens, and to develop skills in Vice-Pres: Ted Rieth [email protected] the lapidary arts. We are members of the Eastern Acting Secretary: JoAnn McGuire [email protected] Federation of Mineralogical & Lapidary Societies (EFMLS) Treasurer & member chair: Trish Benish and the American Federation of Mineralogical Societies [email protected] (AFMS). Immed. Past Pres. Inga Wells [email protected] DUES are payable to the treasurer BY January 1st of each year. After that date membership will be terminated. Make BOARD meetings are held at 6PM on odd-numbered checks payable to Che-Hanna Rock & Mineral Club, Inc. as months unless special meetings are called by the follows: $12.00 for Family; $8.00 for Subscribing Patron; president. $8.00 for Individual and Junior members (under age 17) not BOARD MEMBERS: covered by a family membership. Bruce Benish, Jeff Benish, Mary Walter MEETINGS are held at the Sayre High School (on Lockhart APPOINTED Street) at 7:00 PM in the cafeteria, the 2nd Wednesday Programs: Ted Rieth [email protected] each month, except JUNE, JULY, AUGUST, and Publicity: Hazel Remaley 570-888-7544 DECEMBER. Those meetings and events (and any [email protected] changes) will be announced in this newsletter, with location Editor: David Dick and schedule, as well as on our website [email protected] chehannarocks.com. -

Charlesite, a New Mineral of the Ettringite Group, from Franklin, New Jersey
American Mineralogist, Volume 68, pages 1033-1037, 1983 Charlesite, a new mineral of the ettringite group, from Franklin, New Jersey PETE J. DUNN Department of Mineral Sciences Smithsonian Institution, Washington, D. C. 20560 DONALD R. PEACOR Department of Geological Sciences University of Michigan, Ann Arbor, Michigan 48109 PETER B. LEAVENS Department of Geology University of Delaware, Newark, Delaware 19711 AND JOHN L. BAUM Franklin Mineral Museum Franklin, New Jersey 07416 Abstract Charlesite, ideally C%(AI,Si)2(S04)2(B(OH)4)(OH,0)12.26H20 is a member of the ettrin- gite group from Franklin, New Jersey, and is the Al analogue of sturmanite. Chemical analysis yielded CaO 27.3, Ah03 5.1, Si02 3.1, S03 12.8, B203 3.2, H20 48.6, sum = 100.1 percent. Charlesite is hexagonal, probable space group P31c, with a = 11.16(1), c = 21.21(2)A. The strongest lines in the X-ray powder diffraction pattern (d, 1/10, hkl) are: 9.70, 100, 100; 5.58,80, 110; 3.855, 80, 114; 2.749, 70, 304; 2.538, 70, 126; 2.193, 70, 226/ 404. Charlesite occurs as simple hexagonal crystals tabular on {0001} and has a perfect {10TO}cleavage. The density is 1.77 g/cm3 (obs.) and 1.79 g/cm3 (calc.). Optically, charlesite is uniaxial (-) with w = 1.492(3) and € = 1.475(3). It occurs with clinohedrite, ganophyllite, xonotlite, prehnite, roeblingite and other minerals in several parageneses at Franklin, New Jersey. Charlesite is named in honor of the late Professor Charles Palache. Introduction were approved, prior to publication, by the Commission An ettringite-like mineral was first described from on New Minerals and Mineral Names, I. -

MINERALOGICAL ABSTRACTS from SCIENTIFIC PAPERS PUBLISHED in JAPAN, Edited by the Abstracting Committee, Japan Association of Mineralogical Sciences*
Journal of Mineralogical and Petrological Sciences, Volume 106, page 267-272, 2011 267 MINERALOGICAL ABSTRACTS FROM SCIENTIFIC PAPERS PUBLISHED IN JAPAN, Edited by the Abstracting Committee, Japan Association of Mineralogical Sciences* New Minerals and Mineral Description 106082―106095; 106084 Nitta, E., Kimata, M., Hoshino, M., Echigo. T., Rock-Forming Minerals and Petrology Hamasaki, S., Nishida, N., Shimizu, M. & Akasaka, T. Metamorphic Rocks 106096―106103; Crystal chemistry of ZnS minerals formed as high- temperature volcanic sublimates: matraite identical with Fluid Inclusions 106104―106107; sphalerite. J. Mineral. Petrol. Sci., 103, 145-151, 5 figs., 3 Analytical Methods 106108―106110 tables, 2008. By means of single crystal XRD, EMP, micro XRD and mi- New Minerals and Mineral Description cro-Raman scattering analyses, the crystal chemistry of ZnS min- 106082 Matsubara, S., Mouri, T., Miyawaki, R., Yokoyama, K. erals formed as high-temperature volcanic sublimates from Iwo & Nakahara, M. Munakataite, a new mineral from the dake volcano, Satsuma-Iwojima, Japan, was investigated in detail. Kato mine, Fukuoka, Japan. J. Mineral. Petrol. Sci., 103, They were identified as sphalerite and matraite by using a four- 327-332, 3 figs., 3 tables, 2008. circle automated X-ray diffractometer. However, the two minerals 4+ Munakataite, Pb2Cu2(Se O3)(SO4)(OH)4, occurs as a thin coa were virtually the same in XRD pattern, chemical composition ting on a fracture in a quartz vein containing Cu-Zn-Pb-Ag-Au and micro-Raman scattering spectrum. Close examination of the ore minerals in the Kato mine, Munakata City, Fukuoka observed reflection for the matraite sample showed that all reflec- Prefecture, Japan. -

Carraraite and Zaccagnaite, Two New Minerals from the Carrara Marble Quarries: Their Chemical Compositions, Physical Properties, and Structural Features
American Mineralogist, Volume 86, pages 1293–1301, 2001 Carraraite and zaccagnaite, two new minerals from the Carrara marble quarries: their chemical compositions, physical properties, and structural features STEFANO MERLINO1,* AND PAOLO ORLANDI 1Dipartimento di Scienze della Terra, Università di Pisa, Italy ABSTRACT Two new mineral species, carraraite and zaccagnaite, were found in cavities in calcite veins in marble quarries of the Carrara basin (Apuan Alps, Italy). Carraraite, Ca3Ge(OH)6(SO4)1.08 (CO3)0.92⋅12H2O, occurs as submillimetric crystals, tabular on {001}. The cell dimensions are a = 11.056 (3), c = 10.629 (6) Å, and the space group is P63/m. Carraraite is optically uniaxial (–), ω = 1.509, ε = 1.479. The strongest lines of the X-ray diffraction pattern are at d-spacings (Å): 9.57 (vs) (100), 5.53 (s) (110), 3.83 (s) (112), 3.56 (ms) (202), 2.74 (ms) (302). Carraraite is a new member of the ettringite-thaumasite group, which is characterized by columns of composition [Ca3Ge(OH)6 4+ 2– 2– ⋅12H2O] running along c and interconnected through hydrogen bonding to (SO4) and (CO3) groups. Zaccagnaite, Zn4Al2(OH)12(CO3)⋅3H2O, occurs as minute hexagonal crystals, elongated parallel to [001]. The cell dimensions are a = 3.0725 (3), c = 15.114 (4) Å and the space group is P63/mmc. The crystals are always covered by a thin crust of fraipontite. The strongest lines of the X-ray dif- fraction pattern are at d-spacings (Å): 7.51 (vs) (002), 3.794 (m) (004), 1.542 (ms) (108), 1.539 (ms) (110). -
The Picking Table Volume 42, No. 1
ICKING THE JOURNAL OF THE FRANKLINOGDCNSOURti MINERAL.OGICAL SOCIETY Volume 42, No. 1- Spring / Fall 2001 $15.00 U.S. A Classic Native Copper from Franklin, NJ. Feature: Palache's "Contributions to the Mineralogy of Sterling Hill, New Jersey:" The 900-Foot Level Revisited Schedule of Activities FOMS News The contents of The Picking Table are licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. The Franklin- Ogdensburg Mineraloqical Society, Inc. President William Kroth 240 Union Avenue Committee Chairpersons Wood-Ridge, NJ 07075 Auditing William J. Trost First Vice President Field Trip Edward H. Wilk C. Richard Bieling Warren Cummings (assistant) 315 Oak Street Historical John L. Baum Palmerton, PA 18071 Identification John Cianciulli Mineral Exchange Richard C. Bostwick Second Vice President Nominating Philip P. Betancourt Earl Verbeek Program George Elling 82 Struble Road Spring Swap & Sell Chester S. Lemanski, Jr. Branchville, NJ 07826 Secretary Tema J. Hecht 600 West 111th Street, Apt. 11B Membership Information New York, NY 10025 Anyone interested in the minerals , mines, or mining history Treasurer of the Franklin-Ogdensburg, New Jersey area is invited to John Cianciulli join the Franklin-Ogdensburg Mineralogical Society, Inc. 60 Alpine Road (FOMS). Membership includes scheduled meetings, lec- Sussex, NJ 07461 tures and field trips, as well as a subscription to The Pick- ing Table Assistant Treasurer Steven C. Misiur Membership Rates For One Year: 309 Fernwood Terrace Linden, NJ 07036 $15 Individual Slide Collection Custodian D $20 Family Edward H. Wilk 202 Boiling Springs Avenue East Rutherford, NJ 07073 Name: Trustees Address: John L. Baum (2002) Philip P. -

Theses Digitisation: This Is a Digitised
https://theses.gla.ac.uk/ Theses Digitisation: https://www.gla.ac.uk/myglasgow/research/enlighten/theses/digitisation/ This is a digitised version of the original print thesis. Copyright and moral rights for this work are retained by the author A copy can be downloaded for personal non-commercial research or study, without prior permission or charge This work cannot be reproduced or quoted extensively from without first obtaining permission in writing from the author The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the author When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given Enlighten: Theses https://theses.gla.ac.uk/ [email protected] AN BSOTOPDC AND MINERALOGDCAL DNVESTIGATDON OF CONCRETE DECAY A thesis submitted for the degree of Doctor of Philosophy by Gordon Macleod B.Sc (Hons)(Strathclyde) Department of Geology and Applied Geology, University of Glasgow. June 1990 ProQuest Number: 11007610 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 11007610 Published by ProQuest LLC(2018). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. -

SO3)·11H2O, and the Thaumasiteàhielscherite Solid-Solution Series
Mineralogical Magazine, October 2012, Vol. 76(5), pp. 1133–1152 The sulfite anion in ettringite-group minerals: a new mineral species hielscherite, Ca3Si(OH)6(SO4)(SO3)·11H2O, and the thaumasiteÀhielscherite solid-solution series 1, 2 3 1 4 1 I. V. PEKOV *, N. V. CHUKANOV ,S.N.BRITVIN ,Y.K.KABALOV ,J.GO¨ TTLICHER ,V.O.YAPASKURT , 5 3 6 7 A. E. ZADOV ,S.V.KRIVOVICHEV ,W.SCHU¨ LLER AND B. TERNES 1 Faculty of Geology, Moscow State University, Vorobievy Gory, 119991 Moscow, Russia 2 Institute of Problems of Chemical Physics, Russian Academy of Sciences, Chernogolovka, 142432 Moscow, Russia 3 Faculty of Geology, St Petersburg State University, Universitetskaya Nab. 7/9, St. Petersburg, 199034 Russia, and Nanomaterials Research Centre, Kola Science Center RAS, Fersman Str. 20, 184200 Apatity, Russia 4 Karlsruhe Institute of Technology, Institute for Synchrotron Radiation, Hermann von Helmholtz Platz 1, D-76344 Eggenstein-Leopoldshafen, Germany 5 NPP ‘‘Teplokhim’’, Dmitrovskoye Avenue 71, 127238 Moscow, Russia 6 Im Straubenpesch 22, 53518 Adenau, Germany 7 Bahnhofstrasse 45, 56727 Mayen, Germany [Received 22 April 2012; Accepted 16 July 2012; Associate Editor: Stuart Mills] ABSTRACT Hielscherite, ideally Ca3Si(OH)6(SO4)(SO3)·11H2O, (IMA 2011-037) is the first ettringite-group mineral with essential sulfite. We have identified a continuous natural solid-solution series from endmember thaumasite, Ca3Si(OH)6(SO4)(CO3)·12H2O, to a composition with at least 77 mol.% endmember hielscherite. In this series, the SO3:CO3 ratio is variable, whereas the SO4 content remains constant. Compositions with more than 50 mol.% endmember hielscherite have only been found at Graulay quarry near Hillesheim in the western Eifel Mountains, Rhineland-Palatinate, where they occur with phillipsite-K, chabazite-Ca and gypsum in cavities in alkaline basalt. -
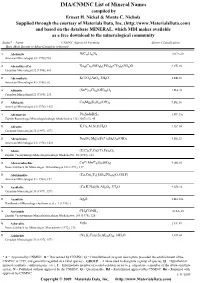
IMA/CNMNC List of Mineral Names
IMA/CNMNC List of Mineral Name s compiled by Ernest H. Nickel & Monte C. Nichols Supplied through the courtesy of Materials Data, Inc. (http://www.MaterialsData.com) and based on the database MINERAL, which MDI makes available as a free download to the mineralogical community Status* Name CNMNC Approved Formula Strunz Classification Best, Most Recent or Most Complete reference. A Abelsonite NiC£¡H£¢N¤ 10.CA.20 American Mineralogist 63 (1978) 930 A Abenakiite-(Ce) Na¢¦Ce¦(SiO£)¦(PO¤)¦(CO£)¦(SO¢)O 9.CK.10 Canadian Mineralogist 32 (1994), 843 G Abernathyite K(UO¢)AsO¤•3H¢O 8.EB.15 American Mineralogist 41 (1956), 82 A Abhurite (SnÀÈ)¢¡Cl¡¦(OH)¡¤O¦ 3.DA.30 Canadian Mineralogist 23 (1985), 233 D Abkhazite Ca¢Mg¥Si¨O¢¢(OH)¢ 9.DE.10 American Mineralogist 63 (1978), 1023 A Abramovite Pb¢SnInBiS§ 2.HF.25a Zapiski Rossiiskogo Mineralogicheskogo Obshchetstva 136 (2007) (5), 45 D Abrazite K,Ca,Al,Si,O,H¢O 9.GC.05 Canadian Mineralogist 35 (1997), 1571 D Abriachanite Na¢(Fe,Mg)£(FeÁÈ)¢Si¨O¢¢(OH)¢ 9.DE.25 American Mineralogist 63 (1978), 1023 D Absite (U,Ca,Y,Ce)(Ti,Fe)¢O¦ Zapiski Vsesoyuznogo Mineralogicheskogo Obshchestva 92 (1963), 113 A Abswurmbachite CuÀÈ(MnÁÈ)¦O¨(SiO¤) 9.AG.05 Neues Jahrbuch für Mineralogie, Abhandlungen 163 (1991), 117 D Abukumalite (Ca,Ce)¢Y£(SiO¤,PO¤)£(O,OH,F) American Mineralogist 51 (1966), 152 D Acadialite (Ca,K,Na)(Si,Al)£O¦•3H¢O 9.GD.10 Canadian Mineralogist 35 (1997), 1571 G Acanthite Ag¢S 2.BA.30a Handbook of Mineralogy (Anthony et al.), 1 (1990), 1 A Acetamide CH£CONH¢ 10.AA.20 Zapiski Vsesoyuznogo Mineralogicheskogo