Bateman, R. (2010) Where Does Orchid Conservation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Genomic Impact of Mycoheterotrophy in Orchids
fpls-12-632033 June 8, 2021 Time: 12:45 # 1 ORIGINAL RESEARCH published: 09 June 2021 doi: 10.3389/fpls.2021.632033 The Genomic Impact of Mycoheterotrophy in Orchids Marcin J ˛akalski1, Julita Minasiewicz1, José Caius2,3, Michał May1, Marc-André Selosse1,4† and Etienne Delannoy2,3*† 1 Department of Plant Taxonomy and Nature Conservation, Faculty of Biology, University of Gdansk,´ Gdansk,´ Poland, 2 Institute of Plant Sciences Paris-Saclay, Université Paris-Saclay, CNRS, INRAE, Univ Evry, Orsay, France, 3 Université de Paris, CNRS, INRAE, Institute of Plant Sciences Paris-Saclay, Orsay, France, 4 Sorbonne Université, CNRS, EPHE, Muséum National d’Histoire Naturelle, Institut de Systématique, Evolution, Biodiversité, Paris, France Mycoheterotrophic plants have lost the ability to photosynthesize and obtain essential mineral and organic nutrients from associated soil fungi. Despite involving radical changes in life history traits and ecological requirements, the transition from autotrophy Edited by: Susann Wicke, to mycoheterotrophy has occurred independently in many major lineages of land Humboldt University of Berlin, plants, most frequently in Orchidaceae. Yet the molecular mechanisms underlying this Germany shift are still poorly understood. A comparison of the transcriptomes of Epipogium Reviewed by: Maria D. Logacheva, aphyllum and Neottia nidus-avis, two completely mycoheterotrophic orchids, to other Skolkovo Institute of Science autotrophic and mycoheterotrophic orchids showed the unexpected retention of several and Technology, Russia genes associated with photosynthetic activities. In addition to these selected retentions, Sean W. Graham, University of British Columbia, the analysis of their expression profiles showed that many orthologs had inverted Canada underground/aboveground expression ratios compared to autotrophic species. Fatty Craig Barrett, West Virginia University, United States acid and amino acid biosynthesis as well as primary cell wall metabolism were among *Correspondence: the pathways most impacted by this expression reprogramming. -
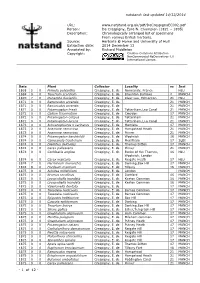
Date Plant Collector Locality Vc Inst 1868 5 0 Primula Polyantha Crespigny, E
natstand: last updated 14/12/2014 URL: www.natstand.org.uk/pdf/DeCrespignyEC002.pdf Person: De Crespigny, Eyre N. Champion (1821 – 1895) Description: Chronologically arranged list of specimens From various British herbaris. Source: Herbaria @ Home and University of Hull Extraction date: 2014 December 13 Annotated by: Richard Middleton Copyright: Creative Commons Attribution- NonCommercial-NoDerivatives 4.0 International License. Date Plant Collector Locality vc Inst 1868 5 0 Primula polyantha Crespigny, E. de Normandy, France HLU 1869 0 0 Teucrium scordium Crespigny, E. de Braunton Burrows 4 MANCH 1870 7 0 Oenanthe fluviatilis Crespigny, E. de River Lee, Edmonton 21 HLU 1871 0 0 Ranunculus arvensis Crespigny, E. de 21 MANCH 1871 0 0 Ranunculus arvensis Crespigny, E. de 21 MANCH 1871 0 0 Potamogeton friesii Crespigny, E. de Tottenham,Lea Canal 21 MANCH 1872 0 0 Galium tricornutum Crespigny, E. de Croydon 17 MANCH 1872 0 0 Potamogeton crispus Crespigny, E. de Tottenham 21 MANCH 1872 0 0 Potamogeton lucens Crespigny, E. de Tottenham,Lea Canal 21 MANCH 1873 0 0 Schoenoplectus x carinatus Crespigny, E. de Mortlake 17 MANCH 1873 0 0 Anemone nemorosa Crespigny, E. de Hampstead Heath 21 MANCH 1873 0 0 Anemone nemorosa Crespigny, E. de Pinner 21 MANCH 1874 0 0 Potamogeton berchtoldii Crespigny, E. de Woolwich 16 MANCH 1874 0 0 Campanula trachelium Crespigny, E. de Merstham 17 SLBI 1874 0 0 Dianthus deltoides Crespigny, E. de Thames Ditton 17 MANCH 1874 0 0 Carex pallescens Crespigny, E. de Pinner 21 MANCH 1874 0 0 Cochlearia anglica Crespigny, E. de Banks of the Thames, 16 HLU Woolwich, London 1874 6 0 Carex vesicaria Crespigny, E. -

Forma Nov.: a New Peloric Orchid from Ibaraki Prefecture, Japan
The Japanese Society for Plant Systematics ISSN 1346-7565 Acta Phytotax. Geobot. 65 (3): 127–139 (2014) Cephalanthera falcata f. conformis (Orchidaceae) forma nov.: A New Peloric Orchid from Ibaraki Prefecture, Japan 1,* 1 1 HirosHi Hayakawa , CHiHiro Hayakawa , yosHinobu kusumoto , tomoko 1 1 2 3,4 nisHida , Hiroaki ikeda , tatsuya Fukuda and Jun yokoyama 1National Institute for Agro-Environmental Sciences, 3-1-3 Kannondai, Tsukuba, Ibaraki 305-8604, Japan. *[email protected] (author for correspondences); 2Faculty of Agriculture, Kochi University, Nan- koku, Kochi 783-8502, Japan; 3Faculty of Science, Yamagata University, Kojirakawa, Yamagata 990-8560, Japan; 4Institute for Regional Innovation, Yamagata University, Kaminoyama, Yamagata 999-3101, Japan We recognize a new peloric form of the orchid Cephalanthera falcata (Thunb.) Blume f. conformis Hi- ros. Hayak. et J. Yokoy., which occurs in the vicinity of the Tsukuba mountain range in Ibaraki Prefec- ture, Japan. This peloric form is sympatric with C. falcata f. falcata. The peloric flowers have a petal-like lip. The flowers are radially symmetrical and the perianth parts spread weakly compared to normal flow- ers. The stigmas are positioned at the column apex, thus neighboring stigmas and the lower parts of the pollinia are conglutinated. We observed similar vegetative traits among C. falcata f. falcata, C. falcata f. albescens S. Kobayashi, and C. falcata f. conformis. The floral morphology in C. falcata f. conformis resembles that of C. nanchuanica (S.C. Chen) X.H. Jin et X.G. Xiang (i.e., similar petal-like lip and stig- ma position at the column apex; syn. Tangtsinia nanchuanica S.C. -

! Natural Potentials of the Medicinal Plants from the Orchidaceae Family with Mucus As the Main Ingredients from Zlatar Mountain
BIOLOGICA NYSSANA 1 (1-2) z December 2010: 43-47 Matović, M. et al. z Natural potentials of the medicinal plants… 1 (1-2) • December 2010: 43-47 10th SFSES • 17-20 June 2010, Vlasina lake Original Article ! Natural potentials of the medicinal plants from the Orchidaceae family with mucus as the main ingredients from Zlatar mountain Milić Matović1, Biljana Nikolić2*, Gorica Đelić3, Marija Marković1 1 University of Niš, Faculty of Sciences and Mathematics, Department of Biology and Ecology, Višegradska 33, 18000 Niš, Serbia 2 Institute of Forestry, Kneza Višeslava 3, 11030 Belgrade, Serbia 3Faculty of Sciences, University of Kragujevac, Radoja Domanovića 12, 34000 Kragujevac, Serbia * E-mail: [email protected] Abstract: Matović, M., Nikolić, B., Đelić, G., Marković, M.: Natural potentials of the medicinal plants from the Orchidaceae family with mucus as the main ingredients from Zlatar mountain. Biologica Nyssana, 1 (1- 2), December 2010: 43-47. The spontaneous medicinal flora of Zlatar Mountain was studied in the aim of realizing the possibilities of its sustainable use for the needs of the pharmaceutical industry. The special attention was paid to genera Orchis, Ophrys, Plathanthera, Gimnadenia, etc. from the orchid family (Orchidaceae) of which salep is made (Tuber salep). Salep is a typical mucous drug (contains over 50% of mucus), which is very beneficial and useful. The primary role of salep is to heal and strengthen the organism and urge the sexual and every other biological ability. Orchids of which salep is made (Orchis coriophora, Orchis laxiflora, Orchis morio, Orchis mascula, Orchis pallens, Orchis purpurea, Orchis simia, Orchis tridentata and Orchis ustulata) are to be found on numerous habitats of Zlatar (in the bright forests, clearing areas and on forest meadows). -

Phytogeographical Analysis and Ecological Factors of the Distribution of Orchidaceae Taxa in the Western Carpathians (Local Study)
plants Article Phytogeographical Analysis and Ecological Factors of the Distribution of Orchidaceae Taxa in the Western Carpathians (Local study) Lukáš Wittlinger and Lucia Petrikoviˇcová * Department of Geography and Regional Development, Faculty of Natural Sciences, Constantine the Philosopher University in Nitra, 94974 Nitra, Slovakia; [email protected] * Correspondence: [email protected]; Tel.: +421-907-3441-04 Abstract: In the years 2018–2020, we carried out large-scale mapping in the Western Carpathians with a focus on determining the biodiversity of taxa of the family Orchidaceae using field biogeographical research. We evaluated the research using phytogeographic analysis with an emphasis on selected ecological environmental factors (substrate: ecological land unit value, soil reaction (pH), terrain: slope (◦), flow and hydrogeological productivity (m2.s−1) and average annual amounts of global radiation (kWh.m–2). A total of 19 species were found in the area, of which the majority were Cephalenthera longifolia, Cephalenthera damasonium and Anacamptis morio. Rare findings included Epipactis muelleri, Epipactis leptochila and Limodorum abortivum. We determined the ecological demands of the abiotic environment of individual species by means of a functional analysis of communities. The research confirmed that most of the orchids that were studied occurred in acidified, calcified and basophil locations. From the location of the distribution of individual populations, it is clear that they are generally arranged compactly and occasionally scattered, which results in ecological and environmental diversity. During the research, we identified 129 localities with the occurrence of Citation: Wittlinger, L.; Petrikoviˇcová, L. Phytogeographical Analysis and 19 species and subspecies of orchids. We identify the main factors that threaten them and propose Ecological Factors of the Distribution specific measures to protect vulnerable populations. -

Impact of Climate Change on the Distribution of Four Closely Related Orchis (Orchidaceae) Species
diversity Article Impact of Climate Change on the Distribution of Four Closely Related Orchis (Orchidaceae) Species Alexandra Evans *, Sam Janssens and Hans Jacquemyn Department of Biology, Plant Conservation and Population Biology, KU Leuven, B-3001 Leuven, Belgium; [email protected] (S.J.); [email protected] (H.J.) * Correspondence: [email protected] Received: 15 July 2020; Accepted: 11 August 2020; Published: 13 August 2020 Abstract: Long-term monitoring programs and population demographic models have shown that the population dynamics of orchids are to a large extent dependent on prevailing weather conditions, suggesting that the changes in climatic conditions can have far reaching effects on the population dynamics and hence the distribution of orchids. Although a better understanding of the effects of climate change on the distribution of plants has become increasingly important during the final years, only a few studies have investigated the effects of changing temperature and precipitation on the distribution of orchids. In this study, we investigated the impact of climate change on the distribution of four terrestrial orchid species (Orchis anthropophora, Orchis militaris, Orchis purpurea and Orchis simia). Using bioclimatic data for current and future climate scenarios, habitat suitability, range shifts and the impact of different abiotic factors on the range of each species were modelled using Maxent. The results revealed an increase in suitable habitat area for O. anthropophora, O. purpurea and O. simia under each RCP (Representative Concentration Pathway) scenario, while a decrease was observed for O. militaris. Furthermore, all four of the orchids showed a shift to higher latitudes under the three RCPs leading to a significant range extension under mild climate change. -

The Charm of the Chiltern Hills
The Charm of the Chiltern Hills Naturetrek Tour Report 16 - 18 May 2018 Man Orchid Burnt Orchid Monkey Orchid Hybrid Monkey x Lady Orchid Report and images compiled by James Harding-Morris Naturetrek Mingledown Barn Wolf's Lane Chawton Alton Hampshire GU34 3HJ UK T: +44 (0)1962 733051 E: [email protected] W: www.naturetrek.co.uk Tour Report The Charm of the Chiltern Hills Tour participants: James Harding-Morris (leader) with three Naturetrek clients Summary This was a three-day tour comprising some of the best orchid and wildflower sites in the Chilterns, a walk along the Thames path from Goring, and a trip to visit RSPB Otmoor for birds. The weather spanned everything from reasonable to excellent, and we certainly made the best of it. Day 1 Wednesday 16th May We met in the bar of the Lambert Arms, introduced ourselves and got down to the business of discussing orchids. This orchid season had been an odd one so far, with several species delayed by the slow start to the year, but others earlier than expected. A couple of these earlier-than-usual species were Burnt Orchid and Man Orchid. As such, we tried something new and headed north to Hoo Bit in Hertfordshire. This patch of woodland and meadow has a lovely mixture of orchids, and it didn’t take us long to spot a number of Fly Orchids – within a few minutes we must have easily seen forty spikes. Twayblades were abundant, as were Common Spotted Orchid rosettes, which hinted at how the meadow must look in high summer. -

The Orchid Garden of Kent
The Orchid Garden of Kent Trip Report 19th May 2018 Led by Jon Dunn Lady Orchid © David Potter Greenwings Wildlife Holidays Tel: 01473 254658 Web: www.greenwings.co.uk Email: [email protected] ©Greenwings 2018 Saturday 19th May dawned bright and sunny in east Kent, and the participants of our inaugural Orchid Garden of Kent day tour gathered in the picturesque village of Wye, nestling at the foot of the chalk downs that bisect Kent and provide pockets of ideal conditions in which some of Britain and Ireland’s rarest and most beautiful orchids can flourish. This was market day, so the village was bustling with people. Happily our transport had arrived before the thronging villagers, so once our guests had arrived we were able to leave the busyness behind us and dive straight into the maze of narrow lanes that meander their way through the surrounding countryside. Spring was in full swing, so the verges were awash with frothing cow parsley punctuated with the pink accents of red campion… but it was altogether rarer flowers that we would be looking for today. Our first stop, a little way outside Wye, was at the edges of Denge Woods. This woodland, managed by the Forestry Commission, contains a gem deep within it – an area renowned amongst orchid-hunters for its remarkable colony of lady orchids Orchis purpurea, a species that is almost entirely restricted to Kent in a British context. On the near continent it may be found, in places, growing with vigorous abandon in swathes on roadside verges but here, in England, it is on the edge of its European range and is altogether rarer. -

(2008) Morphometric and Population Genetic Analyses
Botanical Journal of the Linnean Society, 2008, 157, 687–711. With 11 figures Morphometric and population genetic analyses elucidate the origin, evolutionary significance and conservation implications of Orchis ¥angusticruris (O. purpurea ¥ O. simia), a hybrid orchid new to Britain RICHARD M. BATEMAN*, RHIAN J. SMITH and MICHAEL F. FAY Jodrell Laboratory, Royal Botanic Gardens Kew, Richmond, Surrey TW9 3DS, UK Received 16 January 2008; accepted for publication 17 March 2008 We report the first confirmed occurrence in Britain of Orchis ¥ angusticruris Franch. ex Rouy, a hybrid between two closely related orchid species of anthropomorphic Orchis (O. purpurea Huds. ¥ O. simia Lam.) that hybridize frequently in Continental Europe. Seven individual hybrids, most likely F1 plants representing a single interspe- cific pollination event, first flowered with both parents in May 2006 at a nature reserve in the Chiltern Hills near Goring, Oxfordshire. Univariate and multivariate morphometric analyses (43 characters plus 12 indices), internal transcribed spacer sequencing, plastid microsatellites and amplified fragment length polymorphism (AFLP) analyses together readily separate the parents and confirm that O. purpurea was the ovule parent and O. simia the pollen parent, presumably reflecting the greater frequency and/or later flowering period of the latter at the site. This study reinforces a more general observation that, in most orchids, the ovule parent contributes substantially more to the hybrid phenotype than does the pollen parent, perhaps reflecting cytoplasmic inheritance. In contrast, the hybrids are placed closer to O. simia than to O. purpurea in the AFLP tree. Apparently recent arrivals, the few O. purpurea plants at Goring contrast genetically with the two other small populations of this species known in the Chilterns, but rather are consistent with relatively uncommon Continental populations. -

Orchid Observers
Phenology of UK Plants Orchids and Zooniverse Mark Spencer & Kath Castillo Department of Life Sciences Natural History Museum Agrimonia eupatoria Robbirt & al. 2011 and UK specimens of Ophrys sphegodes Mill NHM Origins and Evolution Initiative: UK Phenology Project • 20,000 herbarium sheets imaged and transcribed • Volunteer contributed taxonomic revision, morphometric and plant/insect pollinator data compiled • Extension of volunteer work to extract additional phenology data from other UK museums and botanic gardens • 7,000 herbarium sheets curated and mounted • Collaboration with BSBI/Herbaria@Home • Preliminary analyses of orchid phenology underway Robbirt & al. (2011) . Validation of biological collections as a source of phenological data for use in climate change studies: a case study with the orchid Ophrys sphegodes. J. Ecol. Brooks, Self, Toloni & Sparks (2014). Natural history museum collections provide information on phenological change in British butterflies since the late-nineteenth century. Int. J. Biometeorol. Johnson & al. (2011) Climate Change and Biosphere Response: Unlocking the Collections Vault. Bioscience. Specimens of Gymnadenia conopsea (L.) R.Br Orchid Observers Phenology of UK Plants Orchids and Zooniverse Mark Spencer & Kath Castillo Department of Life Sciences Natural History Museum 56 species of wild orchid in the UK 29 taxa selected for this study Anacamptis morio Anacamptis pyramidalis Cephalanthera damasonium Coeloglossum viride Corallorhiza trifida Dactylorhiza fuchsii Dactylorhiza incarnata Dactylorhiza maculata Dactylorhiza praetermissa Dactylorhiza purpurella Epipactis palustris Goodyera repens Gymnadenia borealis Gymnadenia conopsea Gymnadenia densiflora Hammarbya paludosa Herminium monorchis Neotinea ustulata Neottia cordata Neottia nidus-avis Neottia ovata Ophrys apifera Ophrys insectifera Orchis anthropophora Orchis mascula Platanthera bifolia Platanthera chlorantha Pseudorchis albida Spiranthes spiralis Fly orchid (Ophrys insectifera) Participants: 1. -

Eurasian Journal of Biological and Chemical Sciences
RESEARCH ARTICLE Eurasian J Bio Chem Sci, 4(1):12-15, 2021 https://doi.org/10.46239/ejbcs.729816 Eurasian Journal of Biological and Chemical Sciences Journal homepage: www.dergipark.org.tr/ejbcs Anatomical Structure and Ecological of Cephalanthera damasonium (Mill.) Druce and Its on Contribution to the Taxonomy of Orchidaceae Derviş ÖZTÜRK1* Eskişehir Osmangazi University, Mahmudiye Equine Vocational School, Department of Plant and Animal Production, Eskişehir, Turkey *Corresponding author : dozturk @ogu.edu.tr Received : 10/04/2020 Orcid No: https://orcid.org/0000-0001-7189-7407 Accepted : 01/04/2021 Abstract: In this study, in the present study reveals the morphological, anatomical and ecological characteristic of Cephalanthera damasonium (Mill.) Druce in Turkey. Plant materials of Cephalanthera species were collected from one population, between 2018 in Eskişehir/Turkey. C. damasonium (Mill.) Druce samples were analyzed for 7 anatomical and soil characters and habitat properties. It was investigated micrometrically in the anatomy of C. damasonium (Mill.) Druce. In morphological investigations, the structure of flower, lateral sepal, petal, dorsal sepal, lip, anther cap and column was determined. The findings were compared with those in Flora of Turkey. to habitat definition, C.damasonium (Mill.) Druce grew up to 800 m to 1200 m. Keywords: Anatomical, Morphological, Cephalanthera damasonium, Eskişehir, Turkey. © EJBCS. All rights reserved. 1. Introduction although tropical areas especially in Asia, Africa and America are the hot spots of diversity. Orchis is a major Orchidaceae is the most famous and attractive plant family genus of Orchidaceae family in Flora of Turkey, among all plant families of the world. Turkey is a rich represented by 22 species (Renz and Taubenheim, 1984; country of terrestrial orchids and represented by 150 taxa Kreutz, 2000). -

Bocconea 25, Results of the Seventh Iter Mediterraneum
Bocconea 25: 5-127 doi: 10.7320/Bocc25.005 Version of Record published online on 9 July 2012 Werner Greuter Results of the Seventh “Iter Mediterraneum” in the Peloponnese, Greece, May to June 1995 (Occasional Papers from the Herbarium Greuter – N° 1) Abstract Greuter, W.: Results of the Seventh “Iter Mediterraneum” in the Peloponnese, Greece, May to June 1995. (Occasional Papers from the Herbarium Greuter – N° 1). — Bocconea. 25: 5-127. 2012. — ISSN 1120-4060 (print), 2280-3882 (online). The material collected during OPTIMA’s Iter Mediterraneum VII to the Peloponnese in 1995 has been revised. It comprises 2708 gatherings, each with 0 to 31 duplicates, collected in 53 numbered localities. The number of taxa (species or subspecies) represented is 1078. As many of the areas visited had been poorly explored before, a dozen of the taxa collected turned out to not to have been previously described, of which 9 (7 species, 2 subspecies) are described and named here (three more were published independently in the intervening years). They belong to the genera Allium, Asperula, Ballota, Klasea, Lolium, Minuartia, Nepeta, Oenanthe, and Trifolium. New combinations at the rank of subspecies (3) and variety (2) are also published. One of the species (Euphorbia aulacosperma) is first recorded for Europe, and several are new for the Peloponnese or had their known range of distribution significantly expanded. Critical notes draw attention to these cases and to taxonomic problems yet to be solved. An overview of the 11 Itinera Mediterranea that have taken place so far is presented, summarising their main results. Keywords: Flora of Greece, Peloponnese, Itinera Mediterranea, OPTIMA, new species, new com- binations, Allium, Asperula, Ballota, Klasea, Lolium, Minuartia, Nepeta, Oenanthe, Trifolium.