Cooking with Chemistry: Candy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Candy Making Made Easy (Because It Is)
05_597345 ch01.qxd 7/29/05 7:08 PM Page 9 Chapter 1 Candy Making Made Easy (Because It Is) Recipes in In This Chapter This Chapter ᮣ Gearing up to make candy ᮣ Dream Dates ᮣ Identifying some great confections ᮣ Checking out special uses for your candy-making skills ne point I stress throughout this book is that candy making is pretty Oeasy. After you learn a few basics and prepare yourself and your envi- ronment according to my simple guidelines, all you have to do is follow a few procedures. I hate to say this, but if I can make candy, you can make candy. When I train new staff members in my candy shop, I observe that, at first, they’re hesitant and overly careful about handling the product, as if it were very fragile. I assure them that they will not hurt the candy by being aggres- sive. I don’t want them to be afraid of the candy, and you shouldn’t be afraid, either. Most products you make are quite tolerant, and you can stir them hard, slap them, or just generally be rough with them. Don’t be afraid to get your hands a little messy, and don’t worry about making a bit of a mess. Have fun, because you’re in charge. To make a point, when someone observes that I have a spot of chocolate on my face, I dab even more on and ask, “Where, here?” Then I touch another spot withCOPYRIGHTED a big dab and ask, “Or was it here?” MATERIAL Before I finish, I have chocolate all over my face, but the new person is relaxed, confident that I am crazy and not worried about making a mess. -

Candy Making Secrets
C a n dy M a ki n g S e cr e t s by MARTIN A . PEASE In which y ou ar e taught to d uplicate AT H OME n ca the fi est ndies m ad e b y any one . C ontaining r ecipes never published r in this fo m b efore. Published by PEASE AND DENISON N ILLI O I ELGl , N S EM RY Of CO NGH QS S Um Games:ti ecesvaci MAY 23 1 908 Gawa i n ; u m : 2 3 f ee 3 CO PY RIGH T , 1908 . PEASE AND DENISON Th e News - Ad vocate n I in i Elgi , ll o s To My WIFE AND BABIES whose fondness of candy led m e to m ake such a success of Hom e Ca ndy M ak n th b k is i g , is oo RESPE CTFULL Y DEDI CA TED By the A uthor INTRODUCTION I I ns N G V N G you the recipes and i tructions contained herein , I have done wh at ~ n o other candym aker ever did to my w a kno ledge , as they always refuse to teach nyone to make candy at home . e m e Aft r teaching a few ladies , the incessant demands on for lessons led me to the writing of this book . It is diff erent from mo st oth er books on H ome Candy M — aking , as I teach you the same method as used by the finest s confectioners , with use of a thermometer , which enable you to always make your candy the same . -

Candy Making Secrets
C a n dy M a ki n g S e cr e t s by MARTIN A . PEASE In which y ou ar e taught to d uplicate AT H OME n ca the fi est ndies m ad e b y any one . C ontaining r ecipes never published r in this fo m b efore. Published by PEASE AND DENISON N ILLI O I ELGl , N S EM RY Of CO NGH QS S Um Games:ti ecesvaci MAY 23 1 908 Gawa i n ; u m : 2 3 f ee 3 CO PY RIGH T , 1908 . PEASE AND DENISON Th e News - Ad vocate n I in i Elgi , ll o s To My WIFE AND BABIES whose fondness of candy led m e to m ake such a success of Hom e Ca ndy M ak n th b k is i g , is oo RESPE CTFULL Y DEDI CA TED By the A uthor INTRODUCTION I I ns N G V N G you the recipes and i tructions contained herein , I have done wh at ~ n o other candym aker ever did to my w a kno ledge , as they always refuse to teach nyone to make candy at home . e m e Aft r teaching a few ladies , the incessant demands on for lessons led me to the writing of this book . It is diff erent from mo st oth er books on H ome Candy M — aking , as I teach you the same method as used by the finest s confectioners , with use of a thermometer , which enable you to always make your candy the same . -

LOLLIPOPS 2014, 1997 by David A
LOLLIPOPS 2014, 1997 by David A. Katz. All rights reserved. Reproduction permitted for education use provided original copyright is included. Materials Needed ½ cup light Karo syrup (corn syrup) 1 cup sugar ½ cup water citric acid (only a “pinch” is needed) flavoring (concentrated candy flavoring preferred – 1/8 teaspoon is needed) food color (concentrated candy food colors preferred – add dropwise) lollipop sticks (available in craft stores) lollipop molds (for hard candies) or baking sheets non-stick cooking spray (Pam or equivalent) stirrer (wood spoon or high temperature candy spoon) 600-mL beaker burner and ring stand or hot plate beaker tongs thermometer (250C or candy thermometer) measuring cups and spoons Safety Safety glasses or goggles must be worn in the laboratory at all times. If this experiment is performed in a chemistry laboratory, all work surfaces must be cleaned and free from laboratory chemicals. After cleaning work surfaces, it is advised to cover all work areas with aluminum foil or a food-grade paper covering. All glassware and apparatus must be clean and free from laboratory chemicals. Use only special glassware and equipment, stored away from all sources of laboratory chemical contamination, and reserved only for food experiments is recommended. There are no safety hazards associated with the materials used in this experiment. Disposal Generally, all waste materials in this experiment can be disposed in the trash or poured down the drain with running water. All disposal must conform to local regulations. Procedure Measure 1 cup sugar into a 600-mL beaker. Add 1/2 cup light Karo syrup. Add 1/2 cup water. -

West Virginia Maple Syrup Producers Association Newsletter
West Virginia Maple Syrup Producers Association Newsletter A Message From Our President July 2017 Dear Members, I would like to begin by thanking Ed Inside This Issue Howell, Mark Bowers and Cathy Hervey for their A Message from Our President..1 service on the executive committee over the past WVMSPA Logo … 2 year and the many people who volunteered their See You at The Fair … 2 time for committee service. I appreciate your 2017 – A Maple Season we will efforts to promote and strengthen our growing association. Never Forget … 2 The 2017 syrup season was another trying season for most of our Dr. Abby’s Workshop … 3 members as we dealt with yet another unusually warm winter and spring. Some Backyard Boling … 3-7 of you may even be thinking “what are we doing, why are we doing this?” As an To Inspect or Not to Inspect? old friend of mine often says… “That’s farming!” We can’t control the weather, That is the Question … 7 but we can control our preparation, quality control, and the many small details Sugar Camp Feature – Blue that go into producing a high quality product like West Virginia Maple Syrup. Even with the 2017 weather we were able to produce over 9,000 gallons of Rock Farm … 8-9 NASS Survey 2017 ….9 finished syrup, 33% more than producers reported for the 2016 season. Turning Your Yellow Leaf The United States, as a whole, added 6% more taps in 2017 versus 2016. Red...10 Our great state of West Virginia added 16% more taps this year, which is the Maple Confections 101 & highest percentage of any state that participated in the USDA National Maple 201...11 Survey. -

Thermal Behavior Characterization of a Sugar-Based Model System and Commercial Confections Across the Stages of Sugar Cooking
THERMAL BEHAVIOR CHARACTERIZATION OF A SUGAR-BASED MODEL SYSTEM AND COMMERCIAL CONFECTIONS ACROSS THE STAGES OF SUGAR COOKING BY MELISSA WANG THESIS Submitted in partial fulfillment of the requirements for the degree of Master of Science in Food Science and Human Nutrition with a concentration in Food Science in the Graduate College of the University of Illinois at Urbana-Champaign, 2017 Urbana, Illinois Adviser: Professor Shelly J. Schmidt Abstract The stages of sugar cooking, although long-existing and widespread in the confection industry, are lacking in thermal behavior profile descriptions, which are crucial to confection functionality. Thermal behavior parameters, such as the glass transition temperature (Tg), are indicative of confection material structure and textural behavior. Tg plays an important role in governing the quality and shelf life of sugar-based confection, and is influenced by moisture content, formulation, and other factors. This study aimed to connect thermal behavior parameters to the stages of sugar cooking. Thus, the objective of this research was to investigate the thermal behavior of the six stages of sugar cooking, as well as representative commercial confections from each stage. A model sugar-based confectionery system was developed and representative commercial confections belonging to each stage of sugar cooking were selected. The model system consisted of a 70:30 ratio of sucrose to corn syrup and a 70:30 ratio of solids to moisture. To investigate the thermal behavior of the stages of sugar cooking, differential scanning calorimetry (DSC), moisture content, and water activity analyses were conducted for the model system and representative commercial confections. The average Tg midpoint of the model system increased from thread to hard crack stage, corresponding to loss of water from increased cooking time and temperature. -
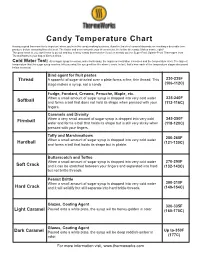
Candy Temperature Chart Having a Good Thermometer Is Important When You’Re in the Candy-Making Business
Candy Temperature Chart Having a good thermometer is important when you’re in the candy-making business. A perfect batch of caramel depends on reaching a desirable tem- perature before removing from the heat. The faster and more accurate your thermometer, the better the candy. Makes sense, right? The good news is, you don’t have to go out and buy a fancy candy thermometer if you’ve already got the Super-Fast, Splash-Proof Thermapen from ThermoWorks in your bag of kitchen tricks. Cold Water Test: As a sugar syrup is cooked, water boils away, the sugar concentration increases and the temperature rises. The highest temperature that the sugar syrup reaches tells you what the syrup will be like when it cools. In fact, that’s how each of the temperature stages discussed below is named. Bind agent for fruit pastes Thread A spoonful of sugar drizzled over a plate forms a fine, thin thread. This 230-235F stage makes a syrup, not a candy (106-112C) Fudge, Fondant, Creams, Penuche, Maple, etc. When a small amount of sugar syrup is dropped into very cold water 235-240F Softball and forms a ball that does not hold its shape when pressed with your (112-116C) fingers. Caramels and Divinity When a very small amount of sugar syrup is dropped into very cold 245-250F Firmball water and forms a ball that holds its shape but is still very sticky when (118-120C) pressed with your fingers. Taffy and Marshmallows When a small amount of sugar syrup is dropped into very cold water 250-265F Hardball and forms a ball that holds its shape but is pliable. -

Holiday Candy and Fudge Collection
Watkins Products and Home Business Opportunity Home Holiday Candy and Fudge Collection Stages of Candy Making Below BEST EVER FUDGE 2 cups of sugar 2 tablespoons of butter 1/3 cup of white Karo syrup 2/3 cup of milk 2 squares of chocolate Watkins Online 1 teaspoon Watkins Vanilla Catalog Sales, specials, recipes, Put all of the above into a heavy saucepan and cook until a soft ball forms (about 5 minutes). gift baskets, gift Beat until slightly thick. Then add any of your favorite nuts. Put in a buttered square cake pan. certificates Let set until cool and cut into squares. Pricelists Watkins Home BUTTERMILK FUDGE Business Opportunity 1 teaspoon of soda Around the Kitchen 1 cup of buttermilk 2 cup of sugar Table - Watkins 2 tablespoon of corn syrup Newsletter 1/2 cup of margarine Subscribe to 1 cup of nuts Around_the_kitchen_table Blend soda and buttermilk, stirring well. Pour sugar into large pan; add buttermilk mixture, corn syrup and margarine. Bring to a boil, cook to 240 degrees F on candy thermometer or to medium soft ball stage. Remove from heat, beat well, stir in nuts. Pour candy into buttered dish or drop by spoonfuls onto waxed paper. VanillaSage Blog BUTTERSCOTCH FUDGE Good Tastings 2 1/2 cups of white sugar Customer Rewards 1 1/2 cup of brown sugar Locate a Watkins 1 stick of butter Associate near you 1/8 teaspoon of salt 1/2 cup of white syrup Facts About Vanilla 1 cup of light cream Cinnamon - The Sweet 1/4 teaspoon of butterscotch flavoring Spice of Life 1 cup of chopped pecans Add Pep to Your Food Combine and cook to a soft ball stage (when dropped in cold water). -

Madness Tour NORTHWEST OHIO
OHIO Maple MADNESS TOUR All roads lead to Ohio Maple Producers during the 2021 Ohio Maple Madness Driving Tour DRIVING TRAIL: MARCH 6 & 7, 13 & 14 2021 OHIO MAPLE PRODUCERS ASSOCIATION Officers: PO Box 1803, President - Karl Evans Burton, Ohio 44021 Vice President - Carly Gavlak [email protected] - Aggie Sojka Sperry Email: Secretary ohiomaple.org Treasurer - Paul Snavely Website: Directors: DISTRICT 1: Paul Snavely, Seneca Co. (2022) Carley Gavlak, Medina Co. (2021) DISTRICT 2: Paul Dierks, Hamilton Co. (2022) *Mark Erlsten, Morrow Co (Appointed) DISTRICT 3: Dan Brown, Knox Co. (2021) Chuck Walker, Licking Co. (2023) DISTRICT 4: Kevin Milligan, Athens Co (2021) *Nate Bissell, Ashtabula Co. (Appointed) DISTRICT 5: Dave Hively, Mahoning Co. (2022) Aggie Sojka Sperry, Geauga Co. (2022) *Denotes Appointed for one year At Large: Jen Freeman, Geauga Co. (2023) Fred Ahrens, Portage Co. (2023) Karl Evans, Ashtabula Co. (2021) Erwin Gingerich III, Geauga Co. (2021) Nathan Goodell, Portage Co. (2023) Website - Nathan Goodell OSU- Dr. Gary Graham Newsletter & OSU - Les Ober Membership - Chuck Walker Ohio Maple Madness Tour NORTHWEST OHIO Maple Moraine Farm Address: 5343 Sandusky Road, Lima, OH 45801 Telephone: 419-233-1552 or 419-221-1552 Email: [email protected] Directions: St Rte 81, 4 miles east of I75(exit127) to Thayer Rd, then north 1 mile to stop sign, then right. ½ mile. Guests welcome anytime. A quaint 200 tap maple operation all gathered with buckets. We grade and bottle all of our maple syrup right off of the evaporator. Taste the sap directly from the tree, then sample the grades of maple syrup fresh off the evaporator. -
How to Make Fondant for Bees
How to Make Fondant for Bees 4 Reasons to Make Bee Fondant, Including Feeding Bees in Winter September 17, 2020 <https://backyardbeekeeping.iamcountryside.com/health-pests/how-to-make-fondant-for-bees/> Reading Time: 6 minutes Fondant for bees is a little different than the fondant that you find at the bakery. The bakery fondant can have high fructose corn syrup, cornstarch, coloring, and flavorings added to it. Making fondant for bees is a lot like making candy. When starting a honey bee farming project, even a small one, itʼs super important to consider the availability of food for the bees. Now, bees are great at finding food but itʼs still wise to intentionally grow plants that attract bees to ensure that they have plenty to eat. However, even with the best planning and intention, there are times that bees might need food from the beekeeper. If you manage your hives well and are diligent to leave enough honey for the bees to make it through the winter or better yet, wait until spring to harvest any honey, you shouldnʼt have to feed your bees very often. When do Bees Need to be Fed? There are several reasons why bees might need to be fed instead of relying solely on what they stored or on foraging. 1. Winter lasts longer normal. No one can predict the future and know exactly how long winter will last or how much honey the bees will eat during the winter. This is the main reason some beekeepers prefer a spring harvest instead of a fall harvest. -

“Let's Make Candies”
CANDY MAKING GUIDE Winnebago County 4-H Mmmmmm… Let’s Make Candies “Candy Can Be More Than Calories” Everyone likes a tasty treat to satisfy their “sweet tooth”, and most often it will be a food containing a large proportion of sugar. Candy is the most concentrated sweet food, but it doesn’t have to provide only “empty”, non- nutritious calories. Many candies that you can make very easily contain ingredients that provide protein, calcium, and other nutrients. Candy should not be a major source of nutrients, as you would have to eat too much of it, but it can be a useful supplement if nutritious recipes are chosen. Watch for such ingredients as peanut butter, oatmeal, nuts, milk, gelatin, and fruit. CANDIES Candies are usually divided into two basic classes: crystalline and non-crystalline. We will also be using three additional classes of candy and candy treats. They are uncooked candies, cereal candies, and microwave candies. Uncooked & Semi-cooked .............................Candies that are uncooked or semi-cooked. Cereal .............................................................Candies that include cereal in the recipe. Crystalline or creamy .....................................Candies that have a distinct crystalline structure such as fondants, fudges, penuche, divinity. Non-crystalline or amorphous .......................Caramels, peanut brittle, butterscotch, hard candy, lollipops, marshmallows, gum drops. Microwave .....................................................Candies that fall in one of the above classes, but are also prepared using a microwave oven. The type of candy made is determined by the ingredients used, the degree of cooking, and the manipulation after cooking. 2 EQUIPMENT No equipment is necessary other than that usually found in the kitchen. Candy-making can become even easier by using certain special equipment, most of which can be obtained at reasonable cost. -

A Brief Discussion of Principles of Candy Making
ing a string in a concentrated cane sugar solution, in which case the sugar is deposited upon the string in crystals gradually increasing in size. In making candy in the home we aim to keep our candy from crystallizing (graining we sometimes call it), else aim to obtain very small crystals, crystals too small to be separately felt. Except in the case of a few simple candies made from confec- tioners' sugar, all candies are cooked. In cooking sugar we may use moist or dry heat. In order to understand thoroughly the principles involved in candy' making, it will be necessary to know the effect of these two forms of heat upon the sugar ordinarily used in candy making, or cane sugar. In order to determine the effect of dry heat upon this sugar the following experiment is rec- ommended. Brittle Candies. Take one-half cup sugar and melt over the fire in a sauce pan, stirring constantly to prevent burning. This should melt completely and be left over the fire with constant stirring until the color just changes, but the material is not burned. This can be poured over whole or crushed peanuts to form peanut brittle. Cocoanut or any other nuts may be substituted, or hot water may be added in sufficient quantities to dissolve this melted sugar and the whole kept for flavoring as caramel. It must be remembered in adding any liquid to the hot melted sugar that the temperature of the melted mass is very high and any cold liquid suddenly intro- duced will tend to harden the mass and make it quite difficult to remove.