LOLLIPOPS 2014, 1997 by David A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Something Sweet Cupcake Flavor List 2017 Name Description
Something Sweet Cupcake Flavor List 2017 Name Description (Some Flavors May Not Be Available All Year) Abigail Cream Cake, Vanilla Bean frosting, topped with a fondant flower Almond-Raspberry Almond Cake, Raspberry filling, Almond/Raspberry swirled frosting, topped w/sliced almonds Aloha Pineapple Cake, Pineapple frosting, topped with Macadamia nuts and toasted coconut AmaZING Yellow Cake, Vanilla filling, Raspberry frosting - Like a Hostess Zinger Apple Pie Apple Cake, Vanilla/ Cinnamon frosting, topped w/ Caramel sauce and a pie crust wafer Banana Cream Banana Cake, Vanilla frosting, topped w/vanilla wafers Banana Loco Banana Cake, Nutella filling, Chocolate/Nutella swirled frostong topped with yellow jimmies and a banana chip Banana Split Banana Cake, Vanilla frosting, topped w/ caramel, chocolate and strawberry sauce plus a cherry on top Beautifully Bavarian Yellow Cake, Chocolate frosting, Bavarian cream filling Better Than Heath Chocolate Cake, Caramel frosting, topped w/Caramel sauce and Heath toffee bits Birthday Cake Vanilla Cake with sprinkles, Tie-dyed Vanilla frosting, topped w/sprinkles Black Bottom Chocolate Fudge Cake filled and iced with Cream Cheese frosting, edged with black sugar crystals Black Forest Dark Chocolate Cake, Vanilla cream filling, Cherry frosting, cherry on top Blackberry Lemon Lemon Cake with Blackberries, Lemon/Blackberry frosting Blueberry Swirl Blueberry cake, blueberry vanilla frosting Boston Cream Pie Yellow Cake, Cream filling, covered with chocolate ganache Bourbon Pecan Bourban flavored chocolate -

Candy Making Made Easy (Because It Is)
05_597345 ch01.qxd 7/29/05 7:08 PM Page 9 Chapter 1 Candy Making Made Easy (Because It Is) Recipes in In This Chapter This Chapter ᮣ Gearing up to make candy ᮣ Dream Dates ᮣ Identifying some great confections ᮣ Checking out special uses for your candy-making skills ne point I stress throughout this book is that candy making is pretty Oeasy. After you learn a few basics and prepare yourself and your envi- ronment according to my simple guidelines, all you have to do is follow a few procedures. I hate to say this, but if I can make candy, you can make candy. When I train new staff members in my candy shop, I observe that, at first, they’re hesitant and overly careful about handling the product, as if it were very fragile. I assure them that they will not hurt the candy by being aggres- sive. I don’t want them to be afraid of the candy, and you shouldn’t be afraid, either. Most products you make are quite tolerant, and you can stir them hard, slap them, or just generally be rough with them. Don’t be afraid to get your hands a little messy, and don’t worry about making a bit of a mess. Have fun, because you’re in charge. To make a point, when someone observes that I have a spot of chocolate on my face, I dab even more on and ask, “Where, here?” Then I touch another spot withCOPYRIGHTED a big dab and ask, “Or was it here?” MATERIAL Before I finish, I have chocolate all over my face, but the new person is relaxed, confident that I am crazy and not worried about making a mess. -

Candy Making Secrets
C a n dy M a ki n g S e cr e t s by MARTIN A . PEASE In which y ou ar e taught to d uplicate AT H OME n ca the fi est ndies m ad e b y any one . C ontaining r ecipes never published r in this fo m b efore. Published by PEASE AND DENISON N ILLI O I ELGl , N S EM RY Of CO NGH QS S Um Games:ti ecesvaci MAY 23 1 908 Gawa i n ; u m : 2 3 f ee 3 CO PY RIGH T , 1908 . PEASE AND DENISON Th e News - Ad vocate n I in i Elgi , ll o s To My WIFE AND BABIES whose fondness of candy led m e to m ake such a success of Hom e Ca ndy M ak n th b k is i g , is oo RESPE CTFULL Y DEDI CA TED By the A uthor INTRODUCTION I I ns N G V N G you the recipes and i tructions contained herein , I have done wh at ~ n o other candym aker ever did to my w a kno ledge , as they always refuse to teach nyone to make candy at home . e m e Aft r teaching a few ladies , the incessant demands on for lessons led me to the writing of this book . It is diff erent from mo st oth er books on H ome Candy M — aking , as I teach you the same method as used by the finest s confectioners , with use of a thermometer , which enable you to always make your candy the same . -

Tablet Press Tooling Press Tablet
Copyright, CSC Publishing. T tablet press tooling Susan Brettingen A tooling vendor’s advice on ablets & Capsules senior editor avoiding common problems The idea to launch a product in tablet form begins in the market- ooks aren’t everything, but first impressions count. ing department, but you’re ultimately responsible for producing it. That’s why it’s important to consider the appearance of Our senior editor talks with a seasoned tooling vendor to identify the product that you plan to tablet. In fact, appearance plays such a vital role in consumer acceptance that the keys to specifying tablet press punches and dies. Much of selecting the tablet’s size, shape, and logo is typically left your success will depend on how well you communicate. toL people in the marketing department. Nonetheless, people in the production department bear the responsi- premature wear on tooling, premature press failure, and Copyright, CSC Publishing. T bility of actually manufacturing the product that the mar- improperly compressed tablets. keting people envisioned. Your ability to do that hinges For example, Natoli said, an engineering department on whether you have the right tooling. Without it, a may buy a tablet press that’s rated at a speed of 4,000 launch delay or cancellation is inevitable. tablets per minute but can only produce 2,700 tablets per That’s not to say that the marketing and production minute in actual production. A situation like this can put departments operate independently of each other. But the a company in a tough spot: Speeding up the press could communication between the two departments is often break the tooling, and investing in another tablet press is not as robust as it should be. -

Hard Candy Lollipop Circle 1.75In Honey Hard Candy
11034 14594 17714 17778 17781 HARD CANDY LOLLIPOP CIRCLE 1.75IN HARD CANDY LOLLIPOP CIRCLE 1.75IN HARD CANDY LOLLIPOP CIRCLE 1.75IN HARD CANDY LOLLIPOP CIRCLE 1.75IN HARD CANDY LOLLIPOP CIRCLE 1.75IN HONEY MINT & TART CHERRY BRANDY ALEXANDER CARAMEL APPLE STRAWBERRY RHUBARB GIN 17782 19367 19659 19664 22318 HARD CANDY LOLLIPOP CIRCLE 1.75IN HARD CANDY LOLLIPOP CIRCLE 1.75IN HARD CANDY LOLLIPOP CIRCLE 1.75IN HARD CANDY LOLLIPOP CIRCLE 1.75IN HARD CANDY LOLLIPOP CIRCLE 1.75IN MAPLE BACON BASIL & POMEGRANATE PEPPERMINT & CACAO CILANTRO LIME GOJI BERRY & ACAI 41016 41017 41019 41020 41021 HARD CANDY LOLLIPOP CIRCLE 1.75IN HARD CANDY LOLLIPOP CIRCLE 1.75IN HARD CANDY LOLLIPOP CIRCLE 1.75IN HARD CANDY LOLLIPOP CIRCLE 1.75IN HARD CANDY LOLLIPOP CIRCLE 1.75IN BEE POLLEN & HONEY BLUEBERRY & ACAI HONEY WITH ORGANIC CHIA SEEDS CHILI CACAO GINGER & COCONUT 41022 41023 41024 41025 41026 HARD CANDY LOLLIPOP CIRCLE 1.75IN HARD CANDY LOLLIPOP CIRCLE 1.75IN HARD CANDY LOLLIPOP CIRCLE 1.75IN HARD CANDY LOLLIPOP CIRCLE 1.75IN HARD CANDY LOLLIPOP CIRCLE 1.75IN GINGER LEMON PUMPKIN & GINGER COCONUT VANILLA MAQUI BERRY & RASPBERRY HONEY & LAVENDER 41027 41028 41174 41234 41644 HARD CANDY LOLLIPOP CIRCLE 1.75IN HARD CANDY LOLLIPOP CIRCLE 1.75IN HARD CANDY LOLLIPOP CIRCLE 1.75IN HARD CANDY LOLLIPOP CIRCLE 1.75IN HARD CANDY LOLLIPOP CIRCLE 1.75IN RASPBERRY LIME GOJI BERRY & CACAO NIB MANGO TIRAMISU & CACAO NIB (CHOCOLATE & CHAMPAGNE BUBBLES COFFEE) WITH SILVER CRYSTALZ 41646 43619 44220 44223 44224 HARD CANDY LOLLIPOP CIRCLE 1.75IN HARD CANDY LOLLIPOP CIRCLE -

Candy Making Secrets
C a n dy M a ki n g S e cr e t s by MARTIN A . PEASE In which y ou ar e taught to d uplicate AT H OME n ca the fi est ndies m ad e b y any one . C ontaining r ecipes never published r in this fo m b efore. Published by PEASE AND DENISON N ILLI O I ELGl , N S EM RY Of CO NGH QS S Um Games:ti ecesvaci MAY 23 1 908 Gawa i n ; u m : 2 3 f ee 3 CO PY RIGH T , 1908 . PEASE AND DENISON Th e News - Ad vocate n I in i Elgi , ll o s To My WIFE AND BABIES whose fondness of candy led m e to m ake such a success of Hom e Ca ndy M ak n th b k is i g , is oo RESPE CTFULL Y DEDI CA TED By the A uthor INTRODUCTION I I ns N G V N G you the recipes and i tructions contained herein , I have done wh at ~ n o other candym aker ever did to my w a kno ledge , as they always refuse to teach nyone to make candy at home . e m e Aft r teaching a few ladies , the incessant demands on for lessons led me to the writing of this book . It is diff erent from mo st oth er books on H ome Candy M — aking , as I teach you the same method as used by the finest s confectioners , with use of a thermometer , which enable you to always make your candy the same . -

West Virginia Maple Syrup Producers Association Newsletter
West Virginia Maple Syrup Producers Association Newsletter A Message From Our President July 2017 Dear Members, I would like to begin by thanking Ed Inside This Issue Howell, Mark Bowers and Cathy Hervey for their A Message from Our President..1 service on the executive committee over the past WVMSPA Logo … 2 year and the many people who volunteered their See You at The Fair … 2 time for committee service. I appreciate your 2017 – A Maple Season we will efforts to promote and strengthen our growing association. Never Forget … 2 The 2017 syrup season was another trying season for most of our Dr. Abby’s Workshop … 3 members as we dealt with yet another unusually warm winter and spring. Some Backyard Boling … 3-7 of you may even be thinking “what are we doing, why are we doing this?” As an To Inspect or Not to Inspect? old friend of mine often says… “That’s farming!” We can’t control the weather, That is the Question … 7 but we can control our preparation, quality control, and the many small details Sugar Camp Feature – Blue that go into producing a high quality product like West Virginia Maple Syrup. Even with the 2017 weather we were able to produce over 9,000 gallons of Rock Farm … 8-9 NASS Survey 2017 ….9 finished syrup, 33% more than producers reported for the 2016 season. Turning Your Yellow Leaf The United States, as a whole, added 6% more taps in 2017 versus 2016. Red...10 Our great state of West Virginia added 16% more taps this year, which is the Maple Confections 101 & highest percentage of any state that participated in the USDA National Maple 201...11 Survey. -

Download and Print the List
Boston Children’s Hospital GI / Nutrition Department 300 Longwood Avenue, Boston, MA 02115 617-355-2127 - CeliacKidsConnection.org This is a list of gluten-free candy by company. Many of your favorite candies may be made by a company you do not associate with that candy. For example, York Peppermint Patties are made by Hershey. If you do not know the parent company, you can often find the name on the product label. In addition, this list is searchable. Open the list in Adobe reader and use the search or magnifying glass icon and search for the name of your favorite candy. Ce De Candy / Smarties Ferrara Candy Co. Continued www.smarties.com • Brach’s Chocolates - Peanut Caramel From the Ce De “Our Candy” Page Clusters, Peanut Clusters, Stars, All Smarties® candy made by Smarties Candy Chocolate Covered Raisins Company is gluten-free and safe for people with • Brach's Double Dipped Peanuts/Double Celiac Disease. Dippers (they are processed in a facility that processes wheat) If the UPC number on the packaging begins with • Brach’s Cinnamon Disks “0 11206”, you can be assured that the product • Brach's Candy Corn - All Varieties is gluten-free, manufactured in a facility that • Brach's Cinnamon Imperials makes exclusively gluten-free products and safe • Brach's Conversation Hearts to eat for people with Celiac Disease. • Brach's Halloween Mellowcremes - All Varieties • Brach's Jelly Bean Nougats Ferrara Candy Company • Brach's Lemon Drops 800-323-1768 • Brach's Wild 'N Fruity Gummi Worms www.ferrarausa.com • Butterfinger (Formerly a Nestle candy) From an email dated 9/15/2020 & 9/18/2020 • Butterfinger bites (Formerly a Nestle Ferrara products contain only Corn Gluten. -

Thermal Behavior Characterization of a Sugar-Based Model System and Commercial Confections Across the Stages of Sugar Cooking
THERMAL BEHAVIOR CHARACTERIZATION OF A SUGAR-BASED MODEL SYSTEM AND COMMERCIAL CONFECTIONS ACROSS THE STAGES OF SUGAR COOKING BY MELISSA WANG THESIS Submitted in partial fulfillment of the requirements for the degree of Master of Science in Food Science and Human Nutrition with a concentration in Food Science in the Graduate College of the University of Illinois at Urbana-Champaign, 2017 Urbana, Illinois Adviser: Professor Shelly J. Schmidt Abstract The stages of sugar cooking, although long-existing and widespread in the confection industry, are lacking in thermal behavior profile descriptions, which are crucial to confection functionality. Thermal behavior parameters, such as the glass transition temperature (Tg), are indicative of confection material structure and textural behavior. Tg plays an important role in governing the quality and shelf life of sugar-based confection, and is influenced by moisture content, formulation, and other factors. This study aimed to connect thermal behavior parameters to the stages of sugar cooking. Thus, the objective of this research was to investigate the thermal behavior of the six stages of sugar cooking, as well as representative commercial confections from each stage. A model sugar-based confectionery system was developed and representative commercial confections belonging to each stage of sugar cooking were selected. The model system consisted of a 70:30 ratio of sucrose to corn syrup and a 70:30 ratio of solids to moisture. To investigate the thermal behavior of the stages of sugar cooking, differential scanning calorimetry (DSC), moisture content, and water activity analyses were conducted for the model system and representative commercial confections. The average Tg midpoint of the model system increased from thread to hard crack stage, corresponding to loss of water from increased cooking time and temperature. -

Ingredients, Equipment and Supplies
Wire Belt Company of America INGREDIENTS, EQUIPMENT Compact Grid is a new belt engineered specifically to han- AND SUPPLIES dle smaller, more delicate prod- ucts such as snack foods, while providing a lighter weight, open-mesh belt design. It has Bradman Lake Inc. Compact Grid 70 percent open area for opti- The Bradman Lake mum cleanability and is ideal for high-speed cooling and Racetrack Cartoner is drying operations. Stainless steel construction. an automated system for Tel: +1 (603) 644 2500 www.wirebelt.com flow-wrapping, collating and packing confec- Aasted-Mikroverk ApS tionery bars into end- ChocoAssort, a direct load cartons. The Race- placing method that track Cartoner collates combines a Frozen- product, then erects, Cone cold-pressing loads and seals endload Racetrack Cartoner platform with a robot cartons at speeds of 260 bars per minute, with an option system, is a complete to increase to 310 ppm. The integrated installation packs concept from Aasted- single pieces or multiple counts of 6, 8 and 12. It can be Mikroverk for mould- used to pack cereal and health bars. ing differently colored ChocoAssort Production System Tel: +1 (704) 588 3301 www.bradmanlake.com and shaped articles in the same pressing operation and subsequently transferring them in the same order direct- Tate & Lyle PLC ly into the boxes. Traditional handling and intermediate Meritab is a new direct compressible dextrose designed storage are eliminated. This method minimizes the risk for use in confectionery products, including candy of blooming, reduces production and cleanup time, and tablets, mints and energy tablets, and pharmaceutical makes zero wastage possible. -

Executive Summary Confectionery Sales Amid COVID-19: 2020 an In-Depth Look at Confectionery Shopping and Consumption During the Pandemic
Executive Summary Confectionery Sales Amid COVID-19: 2020 An in-depth look at confectionery shopping and consumption during the pandemic Part 2 of a 3-part series exploring everyday, seasonal and confectionery shopper insights in a COVID-19 world 1 Copyright© 2020 National Confectioners Association For questions or comments, please contact: All rights reserved. This publication may not be reproduced, stored in any information or . Larry Wilson retrieval system or transmitted in whole or in part, in any form or by any means — Vice President, Industry Affairs electronic, mechanical, photocopying, recording or otherwise — without the express National Confectioners Association written permission of the National Confectioners Association. [email protected] 202.534.1453 . Lynn Wylie Published by: Conducted by: Senior Director, Industry Affairs National Confectioners Association [email protected] 202-534-1466 The National Confectioners Association is the trade organization that promotes the unique role of chocolate, candy, gum and mints in a happy, balanced lifestyle and the companies that make these special treats. Through advocacy and regulatory guidance, communications, industry insights, and retail and supply chain engagement, NCA helps create an environment that enables candy makers and confectionery retailers to thrive. As the leading association for the $36 billion U.S. confectionery industry, NCA works to ensure that chocolate and candy are celebrated for their contributions to culture, society, the economy and everyday moments of joy. Confections are produced in all 50 states, creating jobs for approximately 55,000 workers in more than 1,300 manufacturing facilities across the country. More than 600,000 jobs in agriculture, retail, transportation and other industries rely in part on the sale of confections for their livelihood. -
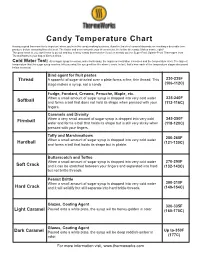
Candy Temperature Chart Having a Good Thermometer Is Important When You’Re in the Candy-Making Business
Candy Temperature Chart Having a good thermometer is important when you’re in the candy-making business. A perfect batch of caramel depends on reaching a desirable tem- perature before removing from the heat. The faster and more accurate your thermometer, the better the candy. Makes sense, right? The good news is, you don’t have to go out and buy a fancy candy thermometer if you’ve already got the Super-Fast, Splash-Proof Thermapen from ThermoWorks in your bag of kitchen tricks. Cold Water Test: As a sugar syrup is cooked, water boils away, the sugar concentration increases and the temperature rises. The highest temperature that the sugar syrup reaches tells you what the syrup will be like when it cools. In fact, that’s how each of the temperature stages discussed below is named. Bind agent for fruit pastes Thread A spoonful of sugar drizzled over a plate forms a fine, thin thread. This 230-235F stage makes a syrup, not a candy (106-112C) Fudge, Fondant, Creams, Penuche, Maple, etc. When a small amount of sugar syrup is dropped into very cold water 235-240F Softball and forms a ball that does not hold its shape when pressed with your (112-116C) fingers. Caramels and Divinity When a very small amount of sugar syrup is dropped into very cold 245-250F Firmball water and forms a ball that holds its shape but is still very sticky when (118-120C) pressed with your fingers. Taffy and Marshmallows When a small amount of sugar syrup is dropped into very cold water 250-265F Hardball and forms a ball that holds its shape but is pliable.