Phys 446: Solid State Physics / Optical Properties
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Atomic Form Factor Calculations of S-States of Helium
American Journal of Modern Physics 2019; 8(4): 66-71 http://www.sciencepublishinggroup.com/j/ajmp doi: 10.11648/j.ajmp.20190804.12 ISSN: 2326-8867 (Print); ISSN: 2326-8891 (Online) Atomic Form Factor Calculations of S-states of Helium Saïdou Diallo *, Ibrahima Gueye Faye, Louis Gomis, Moustapha Sadibou Tall, Ismaïla Diédhiou Department of Physics, Faculty of Sciences, University Cheikh Anta Diop, Dakar, Senegal Email address: *Corresponding author To cite this article: Saïdou Diallo, Ibrahima Gueye Faye, Louis Gomis, Moustapha Sadibou Tall, Ismaïla Diédhiou. Atomic Form Factor Calculations of S-states of Helium. American Journal of Modern Physics . Vol. 8, No. 4, 2019, pp. 66-71. doi: 10.11648/j.ajmp.20190804.12 Received : September 12, 2019; Accepted : October 4, 2019; Published : October 15, 2019 Abstract: Variational calculations of the helium atom states are performed using highly compact 26-parameter correlated Hylleraas-type wave functions. These correlated wave functions used here yield an accurate expectation energy values for helium ground and two first excited states. A correlated wave function consists of a generalized exponential expansion in order to take care of the correlation effects due to N-corps interactions. The parameters introduced in our model are determined numerically by minimization of the total atomic energy of each electronic configuration. We have calculated all integrals analytically before dealing with numerical evaluation. The 1S2 11S and 1 S2S 21, 3 S states energies, charge distributions and scattering atomic form factors are reported. The present work shows high degree of accuracy even with relative number terms in the trial Hylleraas wave functions definition. -
Structure Factors Again
Structure factors again • Remember 1D, structure factor for order h 1 –Fh = |Fh|exp[iαh] = I0 ρ(x)exp[2πihx]dx – Where x is fractional position along “unit cell” distance (repeating distance, origin arbitrary) • As if scattering was coming from a single “center of gravity” scattering point • Presence of “h” in equation means that structure factors of different orders have different phases • Note that exp[2πihx]dx looks (and behaves) like a frequency, but it’s not (dx has to do with unit cell, and the sum gives the phase Back and Forth • Fourier sez – For any function f(x), there is a “transform” of it which is –F(h) = Ûf(x)exp(2pi(hx))dx – Where h is reciprocal of x (1/x) – Structure factors look like that • And it works backward –f(x) = ÛF(h)exp(-2pi(hx))dh – Or, if h comes only in discrete points –f(x) = SF(h)exp(-2pi(hx)) Structure factors, cont'd • Structure factors are a "Fourier transform" - a sum of components • Fourier transforms are reversible – From summing distribution of ρ(x), get hth order of diffraction – From summing hth orders of diffraction, get back ρ(x) = Σ Fh exp[-2πihx] Two dimensional scattering • In Frauenhofer diffraction (1D), we considered scattering from points, along the line • In 2D diffraction, scattering would occur from lines. • Numbering of the lines by where they cut the edges of a unit cell • Atom density in various lines can differ • Reflections now from planes Extension to 3D – Planes defined by extension from 2D case – Unit cells differ • Depends on arrangement of materials in 3D lattice • = "Space -

System Design and Verification of the Precession Electron Diffraction Technique
NORTHWESTERN UNIVERSITY System Design and Verification of the Precession Electron Diffraction Technique A DISSERTATION SUBMITTED TO THE GRADUATE SCHOOL IN PARTIAL FULFILLMENT OF THE REQUIREMENTS for the degree DOCTOR OF PHILOSOPHY Field of Materials Science and Engineering By Christopher Su-Yan Own EVANSTON, ILLINOIS First published on the WWW 01, August 2005 Build 05.12.07. PDF available for download at: http://www.numis.northwestern.edu/Research/Current/precession.shtml c Copyright by Christopher Su-Yan Own 2005 All Rights Reserved ii ABSTRACT System Design and Verification of the Precession Electron Diffraction Technique Christopher Su-Yan Own Bulk structural crystallography is generally a two-part process wherein a rough starting structure model is first derived, then later refined to give an accurate model of the structure. The critical step is the deter- mination of the initial model. As materials problems decrease in length scale, the electron microscope has proven to be a versatile and effective tool for studying many problems. However, study of complex bulk structures by electron diffraction has been hindered by the problem of dynamical diffraction. This phenomenon makes bulk electron diffraction very sensitive to specimen thickness, and expensive equip- ment such as aberration-corrected scanning transmission microscopes or elaborate methodology such as high resolution imaging combined with diffraction and simulation are often required to generate good starting structures. The precession electron diffraction technique (PED), which has the ability to significantly reduce dynamical effects in diffraction patterns, has shown promise as being a “philosopher’s stone” for bulk electron diffraction. However, a comprehensive understanding of its abilities and limitations is necessary before it can be put into widespread use as a standalone technique. -

Form and Structure Factors: Modeling and Interactions Jan Skov Pedersen, Department of Chemistry and Inano Center University of Aarhus Denmark SAXS Lab
Form and Structure Factors: Modeling and Interactions Jan Skov Pedersen, Department of Chemistry and iNANO Center University of Aarhus Denmark SAXS lab 1 Outline • Model fitting and least-squares methods • Available form factors ex: sphere, ellipsoid, cylinder, spherical subunits… ex: polymer chain • Monte Carlo integration for form factors of complex structures • Monte Carlo simulations for form factors of polymer models • Concentration effects and structure factors Zimm approach Spherical particles Elongated particles (approximations) Polymers 2 Motivation - not to replace shape reconstruction and crystal-structure based modeling – we use the methods extensively - alternative approaches to reduce the number of degrees of freedom in SAS data structural analysis (might make you aware of the limited information content of your data !!!) - provide polymer-theory based modeling of flexible chains - describe and correct for concentration effects 3 Literature Jan Skov Pedersen, Analysis of Small-Angle Scattering Data from Colloids and Polymer Solutions: Modeling and Least-squares Fitting (1997). Adv. Colloid Interface Sci. , 70 , 171-210. Jan Skov Pedersen Monte Carlo Simulation Techniques Applied in the Analysis of Small-Angle Scattering Data from Colloids and Polymer Systems in Neutrons, X-Rays and Light P. Lindner and Th. Zemb (Editors) 2002 Elsevier Science B.V. p. 381 Jan Skov Pedersen Modelling of Small-Angle Scattering Data from Colloids and Polymer Systems in Neutrons, X-Rays and Light P. Lindner and Th. Zemb (Editors) 2002 Elsevier -

Direct Phase Determination in Protein Electron Crystallography
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 1791–1794, March 1997 Biophysics Direct phase determination in protein electron crystallography: The pseudo-atom approximation (electron diffractionycrystal structure analysisydirect methodsymembrane proteins) DOUGLAS L. DORSET Electron Diffraction Department, Hauptman–Woodward Medical Research Institute, Inc., 73 High Street, Buffalo, NY 14203-1196 Communicated by Herbert A. Hauptman, Hauptman–Woodward Medical Research Institute, Buffalo, NY, December 12, 1996 (received for review October 28, 1996) ABSTRACT The crystal structure of halorhodopsin is Another approach to such phasing problems, especially in determined directly in its centrosymmetric projection using cases where the structures have appropriate distributions of 6.0-Å-resolution electron diffraction intensities, without in- mass, would be to adopt a pseudo-atom approach. The concept cluding any previous phase information from the Fourier of using globular sub-units as quasi-atoms was discussed by transform of electron micrographs. The potential distribution David Harker in 1953, when he showed that an appropriate in the projection is assumed a priori to be an assembly of globular scattering factor could be used to normalize the globular densities. By an appropriate dimensional re-scaling, low-resolution diffraction intensities with higher accuracy than these ‘‘globs’’ are then assumed to be pseudo-atoms for the actual atomic scattering factors employed for small mol- normalization of the observed structure factors. After this ecule structures -

Solid State Physics: Problem Set #3 Structural Determination Via X-Ray Scattering Due: Friday Jan
Physics 375 Fall (12) 2003 Solid State Physics: Problem Set #3 Structural Determination via X-Ray Scattering Due: Friday Jan. 31 by 6 pm Reading assignment: for Monday, 3.2-3.3 (structure factors for different lattices) for Wednesday, 3.4-3.7 (scattering methods) for Friday, 4.1-4.3 (mechanical properties of crystals) Problem assignment: Chapter 3 Problems: *3.1 Debye-Scherrer analysis (identify the crystal structure) [Robert] 3.2 Lattice parameter from Debye-Scherrer data 3.11 Measuring thermal expansion with X-ray scattering A1. The unit cell dimension of fcc copper is 0.36 nm. Calculate the longest wavelength of X- rays which will produce diffraction from the close packed planes. From what planes could X- rays with l=0.50 nm be diffracted? *A2. Single crystal diffraction: A cubic crystal with lattice spacing 0.4 nm is mounted with its [001] axis perpendicular to an incident X-ray beam of wavelength 0.1 nm. Initially the crystal is set so as to produce a diffracted beam associated with the (020) planes. Calculate the angle through which the crystal must be turned in order to produce a beam from the (hkl) planes where: a) (hkl)=(030) b) (hkl)=(130) [Brian] c) Which of these diffracted beams would be forbidden if the crystal is: i) sc; ii) bcc; iii) fcc A3. Structure factors for the fcc and diamond bases. a) Construct the structure factor Shkl for the fcc lattice and show that it vanishes unless h, k, and l are all even or all odd. b) Construct the structure factor Shkl for the diamond lattice and show that it vanishes unless h, k, and l are all odd or h+k+l=4n where n is an integer. -
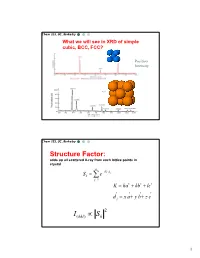
Simple Cubic Lattice
Chem 253, UC, Berkeley What we will see in XRD of simple cubic, BCC, FCC? Position Intensity Chem 253, UC, Berkeley Structure Factor: adds up all scattered X-ray from each lattice points in crystal n iKd j Sk e j1 K ha kb lc d j x a y b z c 2 I(hkl) Sk 1 Chem 253, UC, Berkeley X-ray scattered from each primitive cell interfere constructively when: eiKR 1 2d sin n For n-atom basis: sum up the X-ray scattered from the whole basis Chem 253, UC, Berkeley ' k d k d di R j ' K k k Phase difference: K (di d j ) The amplitude of the two rays differ: eiK(di d j ) 2 Chem 253, UC, Berkeley The amplitude of the rays scattered at d1, d2, d3…. are in the ratios : eiKd j The net ray scattered by the entire cell: n iKd j Sk e j1 2 I(hkl) Sk Chem 253, UC, Berkeley For simple cubic: (0,0,0) iK0 Sk e 1 3 Chem 253, UC, Berkeley For BCC: (0,0,0), (1/2, ½, ½)…. Two point basis 1 2 iK ( x y z ) iKd j iK0 2 Sk e e e j1 1 ei (hk l) 1 (1)hkl S=2, when h+k+l even S=0, when h+k+l odd, systematical absence Chem 253, UC, Berkeley For BCC: (0,0,0), (1/2, ½, ½)…. Two point basis S=2, when h+k+l even S=0, when h+k+l odd, systematical absence (100): destructive (200): constructive 4 Chem 253, UC, Berkeley Observable diffraction peaks h2 k 2 l 2 Ratio SC: 1,2,3,4,5,6,8,9,10,11,12. -

With Synchrotron Radiation Small-Angle X-Ray Scattering (SAXS)
Small-angleSmall-angle X-rayX-ray scatteringscattering (SAXS)(SAXS) withwith synchrotronsynchrotron radiationradiation Martin Müller Institut für Experimentelle und Angewandte Physik der Christian-Albrechts-Universität zu Kiel • Introduction to small-angle scattering • Instrumentation • Examples of research with SAXS Small-angleSmall-angle X-rayX-ray scatteringscattering (SAXS)(SAXS) withwith synchrotronsynchrotron radiationradiation • Introduction to small-angle scattering • Instrumentation • Examples of research with SAXS WhatWhat isis small-anglesmall-angle scattering?scattering? elastic scattering in the vicinity of the primary beam (angles 2θ < 2°) at inhomogeneities (= density fluctuations) typical dimensions in the sample: 0.5 nm (unit cell, X-ray diffraction) to 1 µm (light scattering!) WhatWhat isis small-anglesmall-angle scattering?scattering? pores fibres colloids proteins polymer morphology X-ray scattering (SAXS): electron density neutron scattering (SANS): contrast scattering length OnOn thethe importanceimportance ofof contrastcontrast …… ScatteringScattering contrastcontrast isis relativerelative Babinet‘s principle two different structures may give the same scattering: 2 I(Q) ∝ (ρ1 − ρ2 ) r 4π scattering vector Q = sinθ λ 2θ DiffractionDiffraction andand small-anglesmall-angle scatteringscattering cellulose fibre crystal structure scattering contrast crystals - matrix M. Müller, C. Czihak, M. Burghammer, C. Riekel. J. Appl. Cryst. 33, 817-819 (2000) DiffractionDiffraction andand small-anglesmall-angle scatteringscattering -

Data on the Atomic Form Factor: Computation and Survey Ann T
• Journal of Research of the National Bureau of Standards Vol. 55, No. 1, July 1955 Research Paper 2604 Data on the Atomic Form Factor: Computation and Survey Ann T. Nelms and Irwin Oppenheim This paper presents the results of ca lculations of atomic form factors, based on tables of electron charge distributions compu ted from Hartree wave functions, for a wide range of atomic numbers. Compu tations of t he form factors for fi ve elements- carbon, oxygen, iron, arsenic, and mercury- are presented and a method of interpolation for other atoms is indi cated. A survey of previous results is given and the relativistic theory of Rayleigh scattering is reviewed. Comparisons of the present results with previous computations and with some sparse experimental data are made. 1. Introduction The form factor for an atom of atomic number Z is defined as the matrix: element: The atomic form factor is of interest in the calcu lation of Rayleigh scattering of r adiation and coher en t scattering of charged par ticles from atoms in the region wherc relativist.ic effects can be n eglected. The coheren t scattering of radiation from an atom where 0 denotes the ground state of the atom , and consists of Rayleigh scattering from the electrons, re onant electron scattering, nuclear scattering, and ~ is the vector distance of the jth clectron from the Delbrucl- scattering. When the frequency of the nucleus. For a spherically symmetric atom incident photon approaches a resonant frequency of the atom, large regions of anomalous dispersion occur fC0 = p (r)s i ~rkr dr, (4) in which the form factor calculations are not sufficient 1'" to describe th e coherent scattering. -

CHEM 3030 Introduction to X-Ray Crystallography X-Ray Diffraction Is the Premier Technique for the Determination of Molecular
CHEM 3030 Introduction to X-ray Crystallography X-ray diffraction is the premier technique for the determination of molecular structure in chemistry and biochemistry. There are three distinct parts to a structural determination once a high quality single crystal is grown and mounted on the diffractometer. 1. Geometric data collection – the unit cell dimensions are determined from the angles of a few dozen reflections. 2. Intensity Data Collection – the intensity of several thousand reflections are measured. 3. Structure solution – using Direct or Patterson methods, the phase problem is cracked and a function describing the e-density in the unit cell is generated (Fourier synthesis) from the measured intensities. Least squares refinement then optimizes agreement between Fobs (from Intensity data) and Fcalc (from structure). The more tedious aspects of crystallography have been largely automated and the computations are now within the reach of any PC. Crystallography provides an elegant application of symmetry concepts, mathematics (Fourier series), and computer methods to a scientific problem. Crystal structures are now ubiquitous in chemistry and biochemistry. This brief intro is intended to provide the minimum needed to appreciate literature data. FUNDAMENTALS 1. The 7 crystal systems and 14 Bravais lattices. 2. Space group Tables, special and general positions, translational symmetry elements, screw axes and glide planes. 3. Braggs law. Reflections occur only for integral values of the indices hkl because the distance traveled by an X-ray photon through a unit cell must coincide with an integral number of wavelengths. When this condition is satisfied scattering contributions from all unit cells add to give a net scattered wave with intensity I hkl at an angle Θhkl . -

Solution Small Angle X-Ray Scattering : Fundementals and Applications in Structural Biology
The First NIH Workshop on Small Angle X-ray Scattering and Application in Biomolecular Studies Open Remarks: Ad Bax (NIDDK, NIH) Introduction: Yun-Xing Wang (NCI-Frederick, NIH) Lectures: Xiaobing Zuo, Ph.D. (NCI-Frederick, NIH) Alexander Grishaev, Ph.D. (NIDDK, NIH) Jinbu Wang, Ph.D. (NCI-Frederick, NIH) Organizer: Yun-Xing Wang (NCI-Frederick, NIH) Place: NCI-Frederick campus Time and Date: 8:30am-5:00pm, Oct. 22, 2009 Suggested reading Books: Glatter, O., Kratky, O. (1982) Small angle X-ray Scattering. Academic Press. Feigin, L., Svergun, D. (1987) Structure Analysis by Small-angle X-ray and Neutron Scattering. Plenum Press. Review Articles: Svergun, D., Koch, M. (2003) Small-angle scattering studies of biological macromolecules in solution. Rep. Prog. Phys. 66, 1735- 1782. Koch, M., et al. (2003) Small-angle scattering : a view on the properties, structures and structural changes of biological macromolecules in solution. Quart. Rev. Biophys. 36, 147-227. Putnam, D., et al. (2007) X-ray solution scattering (SAXS) combined with crystallography and computation: defining accurate macromolecular structures, conformations and assemblies in solution. Quart. Rev. Biophys. 40, 191-285. Software Primus: 1D SAS data processing Gnom: Fourier transform of the I(q) data to the P(r) profiles, desmearing Crysol, Cryson: fits of the SAXS and SANS data to atomic coordinates EOM: fit of the ensemble of structural models to SAXS data for disordered/flexible proteins Dammin, Gasbor: Ab initio low-resolution structure reconstruction for SAXS/SANS data All can be obtained from http://www.embl-hamburg.de/ExternalInfo/Research/Sax/software.html MarDetector: 2D image processing Igor: 1D scattering data processing and manipulation SolX: scattering profile calculation from atomic coordinates Xplor/CNS: high-resolution structure refinement GASR: http://ccr.cancer.gov/staff/links.asp?profileid=5546 Part One Solution Small Angle X-ray Scattering: Basic Principles and Experimental Aspects Xiaobing Zuo (NCI-Frederick) Alex Grishaev (NIDDK) 1. -

Problems for Condensed Matter Physics (3Rd Year Course B.VI) A
1 Problems for Condensed Matter Physics (3rd year course B.VI) A. Ardavan and T. Hesjedal These problems are based substantially on those prepared anddistributedbyProfS.H.Simonin Hilary Term 2015. Suggested schedule: Problem Set 1: Michaelmas Term Week 3 • Problem Set 2: Michaelmas Term Week 5 • Problem Set 3: Michaelmas Term Week 7 • Problem Set 4: Michaelmas Term Week 8 • Problem Set 5: Christmas Vacation • Denotes crucial problems that you need to be able to do in your sleep. *Denotesproblemsthatareslightlyharder.‡ 2 Problem Set 1 Einstein, Debye, Drude, and Free Electron Models 1.1. Einstein Solid (a) Classical Einstein Solid (or “Boltzmann” Solid) Consider a single harmonic oscillator in three dimensions with Hamiltonian p2 k H = + x2 2m 2 ◃ Calculate the classical partition function dp Z = dx e−βH(p,x) (2π!)3 ! ! Note: in this problem p and x are three dimensional vectors (they should appear bold to indicate this unless your printer is defective). ◃ Using the partition function, calculate the heat capacity 3kB. ◃ Conclude that if you can consider a solid to consist of N atoms all in harmonic wells, then the heat capacity should be 3NkB =3R,inagreementwiththelawofDulongandPetit. (b) Quantum Einstein Solid Now consider the same Hamiltonian quantum mechanically. ◃ Calculate the quantum partition function Z = e−βEj j " where the sum over j is a sum over all Eigenstates. ◃ Explain the relationship with Bose statistics. ◃ Find an expression for the heat capacity. ◃ Show that the high temperature limit agrees with the law of Dulong of Petit. ◃ Sketch the heat capacity as a function of temperature. 1.2. Debye Theory (a) State the assumptions of the Debye model of heat capacity of a solid.