System Design and Verification of the Precession Electron Diffraction Technique
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Introduction to Phasing Crystallography ISSN 0907-4449
research papers Acta Crystallographica Section D Biological Introduction to phasing Crystallography ISSN 0907-4449 Garry L. Taylor When collecting X-ray diffraction data from a crystal, we Received 30 August 2009 measure the intensities of the diffracted waves scattered from Accepted 22 February 2010 a series of planes that we can imagine slicing through the Centre for Biomolecular Sciences, University of St Andrews, St Andrews, Fife KY16 9ST, crystal in all directions. From these intensities we derive the Scotland amplitudes of the scattered waves, but in the experiment we lose the phase information; that is, how we offset these waves when we add them together to reconstruct an image of our Correspondence e-mail: [email protected] molecule. This is generally known as the ‘phase problem’. We can only derive the phases from some knowledge of the molecular structure. In small-molecule crystallography, some basic assumptions about atomicity give rise to relationships between the amplitudes from which phase information can be extracted. In protein crystallography, these ab initio methods can only be used in the rare cases in which there are data to at least 1.2 A˚ resolution. For the majority of cases in protein crystallography phases are derived either by using the atomic coordinates of a structurally similar protein (molecular replacement) or by finding the positions of heavy atoms that are intrinsic to the protein or that have been added (methods such as MIR, MIRAS, SIR, SIRAS, MAD, SAD or com- binations of these). The pioneering work of Perutz, Kendrew, Blow, Crick and others developed the methods of isomor- phous replacement: adding electron-dense atoms to the protein without disturbing the protein structure. -
Structure Factors Again
Structure factors again • Remember 1D, structure factor for order h 1 –Fh = |Fh|exp[iαh] = I0 ρ(x)exp[2πihx]dx – Where x is fractional position along “unit cell” distance (repeating distance, origin arbitrary) • As if scattering was coming from a single “center of gravity” scattering point • Presence of “h” in equation means that structure factors of different orders have different phases • Note that exp[2πihx]dx looks (and behaves) like a frequency, but it’s not (dx has to do with unit cell, and the sum gives the phase Back and Forth • Fourier sez – For any function f(x), there is a “transform” of it which is –F(h) = Ûf(x)exp(2pi(hx))dx – Where h is reciprocal of x (1/x) – Structure factors look like that • And it works backward –f(x) = ÛF(h)exp(-2pi(hx))dh – Or, if h comes only in discrete points –f(x) = SF(h)exp(-2pi(hx)) Structure factors, cont'd • Structure factors are a "Fourier transform" - a sum of components • Fourier transforms are reversible – From summing distribution of ρ(x), get hth order of diffraction – From summing hth orders of diffraction, get back ρ(x) = Σ Fh exp[-2πihx] Two dimensional scattering • In Frauenhofer diffraction (1D), we considered scattering from points, along the line • In 2D diffraction, scattering would occur from lines. • Numbering of the lines by where they cut the edges of a unit cell • Atom density in various lines can differ • Reflections now from planes Extension to 3D – Planes defined by extension from 2D case – Unit cells differ • Depends on arrangement of materials in 3D lattice • = "Space -

Optical Ptychographic Phase Tomography
University College London Final year project Optical Ptychographic Phase Tomography Supervisors: Author: Prof. Ian Robinson Qiaoen Luo Dr. Fucai Zhang March 20, 2013 Abstract The possibility of combining ptychographic iterative phase retrieval and computerised tomography using optical waves was investigated in this report. The theoretical background and historic developments of ptychographic phase retrieval was reviewed in the first part of the report. A simple review of the principles behind computerised tomography was given with 2D and 3D simulations in the following chapters. The sample used in the experiment is a glass tube with its outer wall glued with glass microspheres. The tube has a diameter of approx- imately 1 mm and the microspheres have a diameter of 30 µm. The experiment demonstrated the successful recovery of features of the sam- ple with limited resolution. The results could be improved in future attempts. In addition, phase unwrapping techniques were compared and evaluated in the report. This technique could retrieve the three dimensional refractive index distribution of an optical component (ideally a cylindrical object) such as an opitcal fibre. As it is relatively an inexpensive and readily available set-up compared to X-ray phase tomography, the technique can have a promising future for application at large scale. Contents List of Figures i 1 Introduction 1 2 Theory 3 2.1 Phase Retrieval . .3 2.1.1 Phase Problem . .3 2.1.2 The Importance of Phase . .5 2.1.3 Phase Retrieval Iterative Algorithms . .7 2.2 Ptychography . .9 2.2.1 Ptychography Principle . .9 2.2.2 Ptychographic Iterative Engine . -

Form and Structure Factors: Modeling and Interactions Jan Skov Pedersen, Department of Chemistry and Inano Center University of Aarhus Denmark SAXS Lab
Form and Structure Factors: Modeling and Interactions Jan Skov Pedersen, Department of Chemistry and iNANO Center University of Aarhus Denmark SAXS lab 1 Outline • Model fitting and least-squares methods • Available form factors ex: sphere, ellipsoid, cylinder, spherical subunits… ex: polymer chain • Monte Carlo integration for form factors of complex structures • Monte Carlo simulations for form factors of polymer models • Concentration effects and structure factors Zimm approach Spherical particles Elongated particles (approximations) Polymers 2 Motivation - not to replace shape reconstruction and crystal-structure based modeling – we use the methods extensively - alternative approaches to reduce the number of degrees of freedom in SAS data structural analysis (might make you aware of the limited information content of your data !!!) - provide polymer-theory based modeling of flexible chains - describe and correct for concentration effects 3 Literature Jan Skov Pedersen, Analysis of Small-Angle Scattering Data from Colloids and Polymer Solutions: Modeling and Least-squares Fitting (1997). Adv. Colloid Interface Sci. , 70 , 171-210. Jan Skov Pedersen Monte Carlo Simulation Techniques Applied in the Analysis of Small-Angle Scattering Data from Colloids and Polymer Systems in Neutrons, X-Rays and Light P. Lindner and Th. Zemb (Editors) 2002 Elsevier Science B.V. p. 381 Jan Skov Pedersen Modelling of Small-Angle Scattering Data from Colloids and Polymer Systems in Neutrons, X-Rays and Light P. Lindner and Th. Zemb (Editors) 2002 Elsevier -

Direct Phase Determination in Protein Electron Crystallography
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 1791–1794, March 1997 Biophysics Direct phase determination in protein electron crystallography: The pseudo-atom approximation (electron diffractionycrystal structure analysisydirect methodsymembrane proteins) DOUGLAS L. DORSET Electron Diffraction Department, Hauptman–Woodward Medical Research Institute, Inc., 73 High Street, Buffalo, NY 14203-1196 Communicated by Herbert A. Hauptman, Hauptman–Woodward Medical Research Institute, Buffalo, NY, December 12, 1996 (received for review October 28, 1996) ABSTRACT The crystal structure of halorhodopsin is Another approach to such phasing problems, especially in determined directly in its centrosymmetric projection using cases where the structures have appropriate distributions of 6.0-Å-resolution electron diffraction intensities, without in- mass, would be to adopt a pseudo-atom approach. The concept cluding any previous phase information from the Fourier of using globular sub-units as quasi-atoms was discussed by transform of electron micrographs. The potential distribution David Harker in 1953, when he showed that an appropriate in the projection is assumed a priori to be an assembly of globular scattering factor could be used to normalize the globular densities. By an appropriate dimensional re-scaling, low-resolution diffraction intensities with higher accuracy than these ‘‘globs’’ are then assumed to be pseudo-atoms for the actual atomic scattering factors employed for small mol- normalization of the observed structure factors. After this ecule structures -

X-Ray Crystallography
X-ray Crystallography Prof. Leonardo Scapozza Pharmceutical Biochemistry School of Pharmaceutical Sciences University of Geneva, University of Lausanne E-mail: [email protected] Aim • Introduce the students to X-ray crystallography • Give the students the tools to “evaluate” a X-ray structure based scientific paper 1 Outline • The History of X-ray • The Principle of X-ray • The Steps towards the 3D structure – Crystallization – X-ray diffraction and data collection – From Pattern of Diffraction to Electron Density – X-ray structure quality assessment An extract of a structure paper 2.1. Crystallization • The hTK1 was cloned as N-terminal thrombin-cleavable His6–tagged fusion protein missing 14 amino acids of the N-terminus and 40 amino acids of the C–terminus of the wild type hTK1 sequence of 234 amino acids (this construct is further on called hTK1). The purified hTK1, consisting of residues 15-194 of the wild type sequence plus an N–terminal extension of 15 residues containing a His6–tag, was eluted from gel filtration column at a concentration of approximate 7 mg/ml with a buffer containing 5 mM Tris at pH 7.2, 10 mM NaCl and 10 mM DTT. For protein crystallization we used the hanging drop method at 23°C. Initial conditions for crystallization were found using Crystal screen Cryo no. 40 (Hampton Research). The protein solution was mixed in a 1:1 ratio with crystallization buffer (0.095 mM tri–sodium citrate pH 5.5, 12% PEG 4000, 10% isopropanol) to set up drops of 6 μl. The reservoir contained 500 μl of crystallization buffer. -

Solid State Physics: Problem Set #3 Structural Determination Via X-Ray Scattering Due: Friday Jan
Physics 375 Fall (12) 2003 Solid State Physics: Problem Set #3 Structural Determination via X-Ray Scattering Due: Friday Jan. 31 by 6 pm Reading assignment: for Monday, 3.2-3.3 (structure factors for different lattices) for Wednesday, 3.4-3.7 (scattering methods) for Friday, 4.1-4.3 (mechanical properties of crystals) Problem assignment: Chapter 3 Problems: *3.1 Debye-Scherrer analysis (identify the crystal structure) [Robert] 3.2 Lattice parameter from Debye-Scherrer data 3.11 Measuring thermal expansion with X-ray scattering A1. The unit cell dimension of fcc copper is 0.36 nm. Calculate the longest wavelength of X- rays which will produce diffraction from the close packed planes. From what planes could X- rays with l=0.50 nm be diffracted? *A2. Single crystal diffraction: A cubic crystal with lattice spacing 0.4 nm is mounted with its [001] axis perpendicular to an incident X-ray beam of wavelength 0.1 nm. Initially the crystal is set so as to produce a diffracted beam associated with the (020) planes. Calculate the angle through which the crystal must be turned in order to produce a beam from the (hkl) planes where: a) (hkl)=(030) b) (hkl)=(130) [Brian] c) Which of these diffracted beams would be forbidden if the crystal is: i) sc; ii) bcc; iii) fcc A3. Structure factors for the fcc and diamond bases. a) Construct the structure factor Shkl for the fcc lattice and show that it vanishes unless h, k, and l are all even or all odd. b) Construct the structure factor Shkl for the diamond lattice and show that it vanishes unless h, k, and l are all odd or h+k+l=4n where n is an integer. -
Simple Cubic Lattice
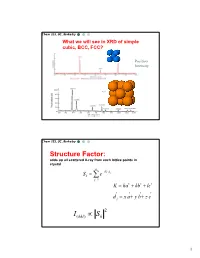
Chem 253, UC, Berkeley What we will see in XRD of simple cubic, BCC, FCC? Position Intensity Chem 253, UC, Berkeley Structure Factor: adds up all scattered X-ray from each lattice points in crystal n iKd j Sk e j1 K ha kb lc d j x a y b z c 2 I(hkl) Sk 1 Chem 253, UC, Berkeley X-ray scattered from each primitive cell interfere constructively when: eiKR 1 2d sin n For n-atom basis: sum up the X-ray scattered from the whole basis Chem 253, UC, Berkeley ' k d k d di R j ' K k k Phase difference: K (di d j ) The amplitude of the two rays differ: eiK(di d j ) 2 Chem 253, UC, Berkeley The amplitude of the rays scattered at d1, d2, d3…. are in the ratios : eiKd j The net ray scattered by the entire cell: n iKd j Sk e j1 2 I(hkl) Sk Chem 253, UC, Berkeley For simple cubic: (0,0,0) iK0 Sk e 1 3 Chem 253, UC, Berkeley For BCC: (0,0,0), (1/2, ½, ½)…. Two point basis 1 2 iK ( x y z ) iKd j iK0 2 Sk e e e j1 1 ei (hk l) 1 (1)hkl S=2, when h+k+l even S=0, when h+k+l odd, systematical absence Chem 253, UC, Berkeley For BCC: (0,0,0), (1/2, ½, ½)…. Two point basis S=2, when h+k+l even S=0, when h+k+l odd, systematical absence (100): destructive (200): constructive 4 Chem 253, UC, Berkeley Observable diffraction peaks h2 k 2 l 2 Ratio SC: 1,2,3,4,5,6,8,9,10,11,12. -

CHEM 3030 Introduction to X-Ray Crystallography X-Ray Diffraction Is the Premier Technique for the Determination of Molecular
CHEM 3030 Introduction to X-ray Crystallography X-ray diffraction is the premier technique for the determination of molecular structure in chemistry and biochemistry. There are three distinct parts to a structural determination once a high quality single crystal is grown and mounted on the diffractometer. 1. Geometric data collection – the unit cell dimensions are determined from the angles of a few dozen reflections. 2. Intensity Data Collection – the intensity of several thousand reflections are measured. 3. Structure solution – using Direct or Patterson methods, the phase problem is cracked and a function describing the e-density in the unit cell is generated (Fourier synthesis) from the measured intensities. Least squares refinement then optimizes agreement between Fobs (from Intensity data) and Fcalc (from structure). The more tedious aspects of crystallography have been largely automated and the computations are now within the reach of any PC. Crystallography provides an elegant application of symmetry concepts, mathematics (Fourier series), and computer methods to a scientific problem. Crystal structures are now ubiquitous in chemistry and biochemistry. This brief intro is intended to provide the minimum needed to appreciate literature data. FUNDAMENTALS 1. The 7 crystal systems and 14 Bravais lattices. 2. Space group Tables, special and general positions, translational symmetry elements, screw axes and glide planes. 3. Braggs law. Reflections occur only for integral values of the indices hkl because the distance traveled by an X-ray photon through a unit cell must coincide with an integral number of wavelengths. When this condition is satisfied scattering contributions from all unit cells add to give a net scattered wave with intensity I hkl at an angle Θhkl . -
Principles of the Phase Contrast (Electron) Microscopy
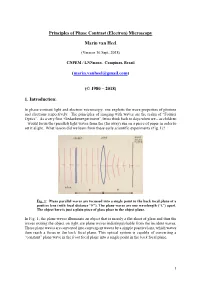
Principles of Phase Contrast (Electron) Microscopy Marin van Heel (Version 16 Sept. 2018) CNPEM / LNNnano, Campinas, Brazil ([email protected]) (© 1980 – 2018) 1. Introduction: In phase-contrast light and electron microscopy, one exploits the wave properties of photons and electrons respectively. The principles of imaging with waves are the realm of “Fourier Optics”. As a very first “Gedankenexperiment”, let us think back to days when we – as children – would focus the (parallel) light waves from the (far away) sun on a piece of paper in order to set it alight. What lesson did we learn from these early scientific experiments (Fig. 1)? Fig. 1: Plane parallel waves are focussed into a single point in the back focal plane of a positive lens (with focal distance “F”). The plane waves are one wavelength (“λ”) apart. The object here is just a plain piece of glass place in the object plane. In Fig. 1, the plane waves illuminate an object that is merely a flat sheet of glass and thus the waves exiting the object on right are plane waves indistinguishable from the incident waves. These plane waves are converted into convergent waves by a simple positive lens, which waves then reach a focus in the back focal plane. This optical system is capable of converting a “constant” plane wave in the front focal plane into a single point in the back focal plane. 1 Fig. 2: A single point scatterer in the object plane leads to secondary (“scattered”) concentric waves emerging from that point in the object. Since the object is placed in the front focal plane, these scattered or “diffracted” secondary waves become plane waves hitting the back focal plane of the system. -
Phase Problem in X-Ray Crystallography, and Its Solution Reciprocal Directions and Spacings from the Real Lattice
Phase Problem in X-ray Secondary article Crystallography, and Its Article Contents . The Phase Problem in X-ray Crystallography Solution . Patterson Methods . Direct Methods Kevin Cowtan, University of York, UK . Multiple Isomorphous Replacement . Multiwavelength Anomalous Dispersion (MAD) X-ray crystallography can provide detailed information about the structure of biological . Molecular Replacement molecules if the ‘phase problem’ can be solved for the molecule under study. The . Phase Improvement phase problem arises because it is only possible to measure the amplitude of diffraction . Summary spots: information on the phase of the diffracted radiation is missing. Techniques are available to reconstruct this information. The Phase Problem in X-ray called the unit cell. It is convenient to define crystal axes a, b Crystallography and c defining the unit cell in three dimensions. Scattering along directions reflecting the lattice repeat will be X-ray diffraction provides one of the most important tools reinforced by every repeat of the unit cell, and will be for examining the three-dimensional (3D) structure of strong; scattering along all other directions will be weak. biological macromolecules. The physics of diffraction As a result, the full diffraction pattern of the crystal is a requires that in order to resolve features of atomic pattern of spots, forming a three-dimensional lattice with structure it is necessary to employ radiation with a wavelength of the order of atomic spacing or smaller. X- rays have suitable wavelength, and interact with the Scattered waves are electrons of the atoms. However, the interaction between out of phase X-rays and electrons is weak, and such energetic radiation causes ionization of the constituent atoms of the molecule, damaging the molecule under study. -

Chapter 3 X-Ray Diffraction • Bragg's Law • Laue's Condition
Chapter 3 X-ray diffraction • Bragg’s law • Laue’s condition • Equivalence of Bragg’s law and Laue’s condition • Ewald construction • geometrical structure factor 1 Bragg’s law Consider a crystal as made out of parallel planes of ions, spaced a distance d apart. The conditions for a sharp peak in the intensity of the scattered radiation are 1. That the x-rays should be specularly reflected by the ions in any one plane and 2. That the reflected rays from successive planes should interfere constructively Path difference between two rays reflected from adjoining planes: 2d sin θ For the rays to interfere constructively, this path difference must be an integral number of wavelength λ nλ = 2d sin θ Bragg’s condition. 2 Bragg angle θ is just the half of the total angle 2 θ by which the incident beam is deflected. There are different ways of sectioning the crystal into planes, each of which will it self produce further reflection. The same portion of Bravais lattice shown in the previous page, with a different way of sectioning the crystal planes. The incident ray is the same. But both the direction and wavelength (determined by Bragg condition with d replaced by d’) of the reflected ray are different from the previous page. 3 Von Laue formulation of X-ray diffraction by a crystal • No particular sectioning of crystal planes r • Regard the crystal as composed of identical microscopic objects placed at Bravais lattice site R • Each of the object at lattice site reradiate the incident radiation in all directions.