Rhamnaceae) in Western Australia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Urticalean Rosids: Circumscription, Rosid Ancestry, and Phylogenetics Based on Rbcl, Trnl-F, and Ndhf Sequences1
American Journal of Botany 89(9): 1531±1546. 2002. URTICALEAN ROSIDS: CIRCUMSCRIPTION, ROSID ANCESTRY, AND PHYLOGENETICS BASED ON RBCL, TRNL-F, AND NDHF SEQUENCES1 KENNETH J. SYTSMA,2,9 JEFFERY MORAWETZ,2,4 J. CHRIS PIRES,2,5 MOLLY NEPOKROEFF,2,6 ELENA CONTI,2,7 MICHELLE ZJHRA,2,8 JOCELYN C. HALL,2 AND MARK W. C HASE3 2Department of Botany, University of Wisconsin, Madison, Wisconsin 53706 USA, and 3Molecular Systematics Section, Royal Botanic Gardens, Kew, UK To address the composition of the urticalean rosids, the relationships of the component families (maximally Cannabaceae, Cecro- piaceae, Celtidaceae, Moraceae, Ulmaceae, and Urticaceae) and analyze evolution of morphological characters, we analyzed sequence variation for a large sampling of these families and various rosid outgroups using rbcL, trnL-F, and ndhF plastid regions. Urticalean rosids are derived out of a lineage including Barbeyaceae, Dirachmaceae, Elaeagnaceae, and Rhamnaceae, with Rosaceae less closely related; thus, they are imbedded within Rosales. Ulmaceae are the sister to all remaining families. Cannabaceae are derived out of a subclade of Celtidaceae; this expanded family should be called Cannabaceae. Cecropiaceae are derived within Urticaceae and are polyphyletic with Poikilospermum derived elsewhere within Urticaceae; this expanded family should be called Urticaceae. Monophy- letic Moraceae are sister to this expanded Urticaceae. Support for these relationships comes from a number of morphological characters (¯oral sexuality, presence or absence of hypanthium, stamen type and dehiscence, pollen pore number, ovule position, and embryo alignment) and chromosome numbers. Most fruit types, in terms of ecological dispersal, are derived independently multiple times and are strongly correlated with habitat. -

Rhamnaceae) Using Scanning Electron Microscopy Jürgen Kellermanna,B
Swainsona 33: 75–102 (2020) © 2020 Board of the Botanic Gardens & State Herbarium (Adelaide, South Australia) A preliminary survey of the leaf-indumentum in the Australian Pomaderreae (Rhamnaceae) using Scanning Electron Microscopy Jürgen Kellermanna,b a State Herbarium of South Australia, GPO Box 1047, Adelaide, South Australia 5001 Email: [email protected] b School of Biological Sciences, Adelaide, The University of Adelaide, South Australia 5005 Abstract: The tribe Pomaderreae (10 genera, c. 240 species) is almost endemic to Australia and defined within Rhamnaceae by the presence of ‘stellate hairs’. This paper is the first to report observations on the leaf-indumentum of 33 species of the tribe with Scanning Electron Microscopy. Apart from simple hairs and papillae, three types of stellate trichomes were observed: fasciculate hairs, appressed stellate hairs and multiradiate hairs (sessile or stalked). The existence of stellate trichomes on the examined species of Pomaderreae was confirmed. Trichomes and indumenta are often variable within genera, as the type of indumentum may be more related to its function. Some groups of species in the genera Cryptandra Sm., Pomaderris Labill. and Stenanthemum Reissek have indumentum characters that seem to be more consistent and might be of value to elucidate relationships between taxa. Keywords: Rhamnaceae, Pomaderreae, leaf morphology, trichomes, hairs, leaf surface, SEM Introduction xeromorphic adaptations, such as small, hard, revolute or conduplicate leaves, a dense indumentum or spines. Pomaderreae Reissek ex Endl. is the second largest tribe of Rhamnaceae. It was first described by Reissek On-going molecular and morphological studies in (1840), but not accepted by subsequent botanists (e.g. -

Rhamnaceae) Jürgen Kellermanna,B
Swainsona 33: 43–50 (2020) © 2020 Board of the Botanic Gardens & State Herbarium (Adelaide, South Australia) Nomenclatural notes and typifications in Australian species of Paliureae (Rhamnaceae) Jürgen Kellermanna,b a State Herbarium of South Australia, GPO Box 1047, Adelaide, South Australia 5001 Email: [email protected] b The University of Adelaide, School of Biological Sciences, Adelaide, South Australia 5005 Abstract: The nomenclature of the four species of Ziziphus Mill. and the one species of Hovenia Thunb. occurring in Australia is reviewed, including the role of the Hermann Herbarium for the typification of Z. oenopolia (L.) Mill. and Z. mauritiana Lam. Lectotypes are chosen for Z. quadrilocularis F.Muell. and Z. timoriensis DC. A key to species is provided, as well as illustrations for Z. oenopolia, Z. quadrilocularis and H. dulcis Thunb. Keywords: Nomenclature, typification, Hovenia, Ziziphus, Rhamnaceae, Paliureae, Paul Hermann, Carolus Linnaeus, Henry Trimen, Australia Introduction last worldwide overview of the genus was published by Suessenguth (1953). Since then, only regional Rhamnaceae tribe Paliureae Reissek ex Endl. was treatments and revisions have been published, most reinstated by Richardson et al. (2000b), after the first notably by Johnston (1963, 1964, 1972), Bhandari & molecular analysis of the family (Richardson et al. Bhansali (2000), Chen & Schirarend (2007) and Cahen 2000a). It consists of three genera, Hovenia Thunb., et al. (in press). For Australia, the genus as a whole was Paliurus Tourn. ex Mill. and Ziziphus Mill., which last reviewed by Bentham (1863), with subsequent until then were assigned to the tribes Rhamneae regional treatments by Wheeler (1992) and Rye (1997) Horan. -

Phylogenetic Analysis of Vitaceae Based on Plastid Sequence Data
PHYLOGENETIC ANALYSIS OF VITACEAE BASED ON PLASTID SEQUENCE DATA by PAUL NAUDE Dissertation submitted in fulfilment of the requirements for the degree MAGISTER SCIENTAE in BOTANY in the FACULTY OF SCIENCE at the UNIVERSITY OF JOHANNESBURG SUPERVISOR: DR. M. VAN DER BANK December 2005 I declare that this dissertation has been composed by myself and the work contained within, unless otherwise stated, is my own Paul Naude (December 2005) TABLE OF CONTENTS Table of Contents Abstract iii Index of Figures iv Index of Tables vii Author Abbreviations viii Acknowledgements ix CHAPTER 1 GENERAL INTRODUCTION 1 1.1 Vitaceae 1 1.2 Genera of Vitaceae 6 1.2.1 Vitis 6 1.2.2 Cayratia 7 1.2.3 Cissus 8 1.2.4 Cyphostemma 9 1.2.5 Clematocissus 9 1.2.6 Ampelopsis 10 1.2.7 Ampelocissus 11 1.2.8 Parthenocissus 11 1.2.9 Rhoicissus 12 1.2.10 Tetrastigma 13 1.3 The genus Leea 13 1.4 Previous taxonomic studies on Vitaceae 14 1.5 Main objectives 18 CHAPTER 2 MATERIALS AND METHODS 21 2.1 DNA extraction and purification 21 2.2 Primer trail 21 2.3 PCR amplification 21 2.4 Cycle sequencing 22 2.5 Sequence alignment 22 2.6 Sequencing analysis 23 TABLE OF CONTENTS CHAPTER 3 RESULTS 32 3.1 Results from primer trail 32 3.2 Statistical results 32 3.3 Plastid region results 34 3.3.1 rpL 16 34 3.3.2 accD-psa1 34 3.3.3 rbcL 34 3.3.4 trnL-F 34 3.3.5 Combined data 34 CHAPTER 4 DISCUSSION AND CONCLUSIONS 42 4.1 Molecular evolution 42 4.2 Morphological characters 42 4.3 Previous taxonomic studies 45 4.4 Conclusions 46 CHAPTER 5 REFERENCES 48 APPENDIX STATISTICAL ANALYSIS OF DATA 59 ii ABSTRACT Five plastid regions as source for phylogenetic information were used to investigate the relationships among ten genera of Vitaceae. -

Ceanothus Crassifolius Torrey NRCS CODE: Family: Rhamnaceae (CECR) Order: Rhamnales Subclass: Rosidae Class: Magnoliopsida
I. SPECIES Ceanothus crassifolius Torrey NRCS CODE: Family: Rhamnaceae (CECR) Order: Rhamnales Subclass: Rosidae Class: Magnoliopsida Lower right: Ripening fruits, two already dehisced. Lower center: Longitudinal channeling in stems of old specimen, typical of obligate seeding Ceanothus (>25 yr since last fire). Note dark hypanthium in center of white flowers. Photos by A. Montalvo. A. Subspecific taxa 1. C. crassifolius Torr. var. crassifolius 2. C. crassifolius Torr. var. planus Abrams (there is no NRCS code for this taxon) B. Synonyms 1. C. verrucosus Nuttal var. crassifolius K. Brandegee (Munz & Keck 1968; Burge et al. 2013) 2. C. crassifolius (in part, USDA PLANTS 2019) C. Common name 1. hoaryleaf ceanothus, sometimes called thickleaf ceanothus or thickleaf wild lilac (Painter 2016) 2. same as above; flat-leaf hoary ceanothus and flat-leaf snowball ceanothus are applied to other taxa (Painter 2016) D. Taxonomic relationships Ceanothus is a diverse genus with over 50 taxa that cluster in to two subgenera. C. crassifolius has long been recognized as part of the Cerastes group of Ceanothus based on morphology, life-history, and crossing studies (McMinn 1939a, Nobs 1963). In phylogenetic analyses based on RNA and chloroplast DNA, Hardig et al. (2000) found C. crassifolius clustered into the Cerastes group and in each analysis shared a clade with C. ophiochilus. In molecular and morphological analyses, Burge et al. (2011) also found C. crassifolius clustered into Cerastes. Cerastes included over 20 taxa and numerous subtaxa in both studies. Eight Cerastes taxa occur in southern California (see I. E. Related taxa in region). E. Related taxa in region In southern California, the related Cerastes taxa include: C. -
Vegetation and Ecological Characterisitics of Mixed-Conifer
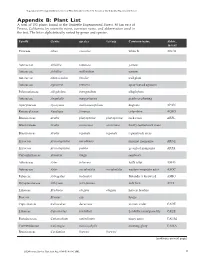
Vegetation and Ecological Charactistics of Mixed-Conifer and Red Fir Forests at the Teakettle Experimental Forest Vegetation and Ecological Characteristics of Mixed-Conifer and Red Fir Forests at the Teakettle Experimental Forest Appendix B: Plant List A total of 152 plants found at the Teakettle Experimental Forest, 80 km east of Fresno, California, by scientific name, common name, and abbreviation used in the text. The list is alphabetically sorted by genus and species. Family Genus species var/ssp Common name Abbre. in text Pinaceae Abies concolor white fir ABCO Pinaceae Abies magnifica red fir ABMA Asteraceae Achillea lanulosa yarrow Asteraceae Achillea millefolium yarrow Asteraceae Adenocaulon bicolor trail plant Asteraceae Agroseris retrorsa spear-leaved agoseris Polemoniaceae Allophylum intregifolium allophylum Asteraceae Anaphalis margaritacea pearly everlasting Apocynaceae Apocynum androsaemifolium dogbane APAN Ranunculaceae Aquilegia formosa columbine AQFO Brassicaceae Arabis platysperma platysperma rock cress ARPL Brassicaceae Arabis rectissima rectissima bristly-leaved rock cress Brassicaceae Arabis repanda repanda repand rock cress Ericaceae Arctostaphylus nevadensis pinemat manzanita ARNE Ericaceae Arctostaphylus patula greenleaf manzanita ARPA Caryophyliaceae Arenaria kingii sandwort Asteraceae Aster foliaceus leafy aster ASFO Asteraceae Aster occidentalis occidentalis western mountain aster ASOC Fabaceae Astragalus bolanderi Bolander’s locoweed ASBO Dryopteridaceae Athryium felix-femina lady fern ATFI Liliaceae Brodiaea elegans elegans harvest brodeia Poaceae Bromus ssp. brome Cupressaceae Calocedrus decurrens incense cedar CADE Liliaceae Calochortus leichtlinii Leichtlin’s mariposa lily CALE Portulacaceae Calyptridium umbellatum pussy paws CAUM Convuvulaceae Calystegia malacophylla morning glory CAMA Brassicaceae Cardamine breweri breweri (continues on next page) 46 USDA Forest Service Gen.Tech. Rep. PSW-GTR-186. 2002. USDA Forest Service Gen.Tech. Rep. -

A Phylogenetic Analysis of Rhamnaceae Using Rbcl and Trnl-F Plastid DNA Sequences James E. Richardson
A Phylogenetic Analysis of Rhamnaceae using rbcL and trnL-F Plastid DNA Sequences James E. Richardson; Michael F. Fay; Quentin C. B. Cronk; Diane Bowman; Mark W. Chase American Journal of Botany, Vol. 87, No. 9. (Sep., 2000), pp. 1309-1324. Stable URL: http://links.jstor.org/sici?sici=0002-9122%28200009%2987%3A9%3C1309%3AAPAORU%3E2.0.CO%3B2-5 American Journal of Botany is currently published by Botanical Society of America. Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/about/terms.html. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/journals/botsam.html. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. The JSTOR Archive is a trusted digital repository providing for long-term preservation and access to leading academic journals and scholarly literature from around the world. The Archive is supported by libraries, scholarly societies, publishers, and foundations. It is an initiative of JSTOR, a not-for-profit organization with a mission to help the scholarly community take advantage of advances in technology. -

The Genus Ceanothus: Wild Lilacs and Their Kin
THE GENUS CEANOTHUS: WILD LILACS AND THEIR KIN CEANOTHUS, A GENUS CENTERED IN CALIFORNIA AND A MEMBER OF THE BUCKTHORN FAMILY RHAMNACEAE The genus Ceanothus is exclusive to North America but the lyon’s share of species are found in western North America, particularly California • The genus stands apart from other members of the Rhamnaceae by • Colorful, fragrant flowers (blues, purples, pinks, and white) • Dry, three-chambered capsules • Sepals and petals both colored and shaped in a unique way, and • Three-sided receptacles that persist after the seed pods have dropped Ceanothus blossoms feature 5 hooded sepals, 5 spathula-shaped petals, 5 stamens, and a single pistil with a superior ovary Ceanothus seed pods are three sided and appear fleshy initially before drying out, turning brown, and splitting open Here are the three-sided receptacles left behind when the seed pods fall off The ceanothuses range from low, woody ground covers to treelike forms 20 feet tall • Most species are evergreen, but several deciduous kinds also occur • Several species feature thorny side branches • A few species have highly fragrant, resinous leaves • Habitats range from coastal bluffs through open woodlands & forests to chaparral and desert mountains The genus is subdivided into two separate subgenera that seldom exchange genes, even though species within each subgenus often hybridize • The true ceanothus subgenus (simply called Ceanothus) is characterized by • Alternate leaves with deciduous stipules • Leaves that often have 3 major veins (some have a single prominent midrib) • Flowers mostly in elongated clusters • Smooth seed pods without horns • The majority of garden species available belong to this group The leaves of C. -

Rhamnaceae Buckthorn
Rhamnus spp. Family: Rhamnaceae Buckthorn The genus Rhamnus contains over 100 species native to: North America [5], the rest from the north temperate regions, South America and South Africa. Many non-native species have been naturalized in the US. The name rhamnus is an ancient Greek name. Rhamnus alaternus-Mediterranean Buckthorn (Europe) Rhamnus alpinus-Alpine Buckthorn (Europe) Rhamnus betulifolia-Birchleaf Buckthorn Rhamnus californica-California Buckthorn , California Coffeeberry, Coast Coffeeberry, Coffeeberry, Pigeonberry, Sierra Coffeeberry Rhamnus caroliniana-Alder Buckthorn, Birch Bog, Brittlewood, Buckthorn-tree, Carolina Buckthorn , Elbow-brush, Indian Cherry, Pale-cat-wood, Polecat-tree, Polecatwood, Stinkberry, Stink Cherry, Stinkwood, Tree Buckthorn, Yellow Buckthorn, Yellowwood Rhamnus catharticus-Common Buckthorn, European Buckthorn , European Waythorn, Purgin Buckthorn Rhamnus crocea-California Redberry, Coffeeberry, Evergreen Buckthorn, Great Redberry Buckthorn, Hollyleaf Buckthorn , Island Buckthorn, Island Redberry Buckthorn, Redberry, Redberry Buckthorn Rhamnus frangula-(Europe) Alder Buckthorn, Glossy Buckthorn Rhamnus purshiana*-Bayberry, Bearberry, Bearwood, Bitterbark, Bitterboom, Bittertrad, Buckthorn Cascara, California Coffee, Cascara, Cascara Buckthorn , Cascara Sagrada, Chitam, Chittam, Chittern, Chittim, Coffeeberry, Coffeebush, Coffeetree, Oregon Bearwood, Pigeonberry, Shittimwood, Wahoo, Western Coffee, Wild Cherry, Wild Coffee, Wild Coffeebush, Yellow-wood Rhamnus zeyheri-(Africa) Pink Ivory, Red Ivorywood *commercial American species The following is for Cascara Buckthorn: Distribution The Pacific Coast region from British Columbia (incl. Vancouver Island), south to Washington, Oregon and northern California in Coast Ranges and the Sierra Nevada. Also in the Rocky Mountain region of British Columbia, Washington Idaho and Montana. The Tree Cascara Buckthorn grows in bottom lands, but can be found along fence rows and roadsides. It grows scattered among Douglas fir, maples, western redcedar and hemlock. -

Vascular Plant Families of the United States Grouped by Diagnostic Features
Humboldt State University Digital Commons @ Humboldt State University Botanical Studies Open Educational Resources and Data 12-6-2019 Vascular Plant Families of the United States Grouped by Diagnostic Features James P. Smith Jr Humboldt State University, [email protected] Follow this and additional works at: https://digitalcommons.humboldt.edu/botany_jps Part of the Botany Commons Recommended Citation Smith, James P. Jr, "Vascular Plant Families of the United States Grouped by Diagnostic Features" (2019). Botanical Studies. 96. https://digitalcommons.humboldt.edu/botany_jps/96 This Flora of the United States and North America is brought to you for free and open access by the Open Educational Resources and Data at Digital Commons @ Humboldt State University. It has been accepted for inclusion in Botanical Studies by an authorized administrator of Digital Commons @ Humboldt State University. For more information, please contact [email protected]. FLOWERING PLANT FAMILIES OF THE UNITED STATES GROUPED BY DIAGNOSTIC FEATURES James P. Smith, Jr. Professor Emeritus of Botany Department of Biological Sciences Humboldt State University Second edition — 6 December 2019 The focus is on families of plants found in the conterminous United States, including ornamentals. The listing of a family is not meant to imply that every species has that feature. I am using a fewfamily names, such as Liliaceae, Plantaginaceae, and Scrophulariaceae, in the traditional sense, because their limits remain unsettled. Parasitic on branches Dioscoreaceae -

Western Australia's Journal of Systematic
WESTERN AUSTRALIA’S JOURNAL OF SYSTEMATIC BOTANY ISSN 0085-4417 Hopper, S.D. & Wardell-Johnson, G. Eucalyptus virginea and E. relicta (Myrtaceae), two new rare forest trees from south-western Australia allied to E. lane-poolei, and a new phantom hybrid. Nuytsia 15(2): 227–240 (2004) All enquiries and manuscripts should be directed to: The Editor – NUYTSIA Western Australian Herbarium Telephone: +61 8 9334 0500 Conservation and Land Management Facsimile: +61 8 9334 0515 Locked Bag 104 Bentley Delivery Centre Email: [email protected] Western Australia 6983 Web: science.calm.wa.gov.au/nuytsia/ AUSTRALIA All material in this journal is copyright and may not be reproduced except with the written permission of the publishers. © Copyright Department of Conservation and Land Management . S.D.Nuytsia Hopper 15(2):227–240(2004) and G. Wardell-Johnson, Two new rare forest trees from south-western Australia 227 Eucalyptus virginea and E. relicta (Myrtaceae), two new rare forest trees from south-western Australia allied to E. lane-poolei, and a new phantom hybrid Stephen D. Hopper1 and Grant Wardell-Johnson2 1School of Plant Biology, The University of Western Australia, Crawley, Western Australia 6907 2School of Natural and Rural Systems Management, The University of Queensland, Gatton, Queensland 4343 Abstract Hopper, S.D. & Wardell-Johnson, G. Eucalyptus virginea and E. relicta (Myrtaceae), two new rare forest trees from south-western Australia allied to E. lane-poolei, and a new phantom hybrid. Nuytsia 15(2): 227–240 (2004). Eucalyptus virginea and E. relicta are described from Mt Lindesay National Park and from the Whicher Range south-east of Busselton respectively. -

Nomenclatural Notes on the Alphitonia Group in Australia (Rhamnaceae) Jürgen Kellermanna,B
Swainsona 33: 135–142 (2020) © 2020 Board of the Botanic Gardens & State Herbarium (Adelaide, South Australia) Nomenclatural notes on the Alphitonia Group in Australia (Rhamnaceae) Jürgen Kellermanna,b a State Herbarium of South Australia, Botanic Gardens and State Herbarium, Hackney Road, Adelaide, South Australia 5000 Email: [email protected] b The University of Adelaide, School of Biological Sciences, Adelaide, South Australia 5005 Abstract: The nomenclature and typification of seven species of Alphitonia Reissek ex Endl. and Emmenosperma F.Muell. is discussed. These two genera form the “Alphitonia Group” together with Granitites Rye and Jaffrea H.C.Hopkins & Pillon from New Caledonia. Lectotypes are chosen for E. cunninghamii Benth. and for the synonyms A. excelsa var. acutifolia Braid, A. obtusifolia R.Br. ex Braid and A. obtusifolia var. tenuis Braid. The lectotypes of A. petriei Braid & C.T.White and A. philippinensis Braid are clarified. Three species are illustrated: A. petriei, A. whitei Braid and E. cunninghamii. Keywords: Nomenclature, typification, second-step lectotype, Rhamnaceae, incertae sedis, Australia Introduction when fruits are ripe, they split open and the pericarp falls off, leaving the seeds (usually 3) remaining attached Rhamnaceae Juss. is a medium-sized plant family with to the base of the fruit (Fig. 3F, P). over 1000 species in 64 genera. The current intra- familial classification was devised by Richardson et al. Alphitonia (10–15 species) was published in (2000b), following the first molecular analysis of the Endlicher (1840), using Reissek’s manuscript name whole family (Richardson et al. 2000a). The family is and description. He indicated that Colubrina excelsa divided into 11 tribes, but several genera have not been A.Cunn.