Rhamnus Alaternus Plant: Extraction of Bioactive Fractions and Evaluation of Their Pharmacological and Phytochemical Properties
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Urticalean Rosids: Circumscription, Rosid Ancestry, and Phylogenetics Based on Rbcl, Trnl-F, and Ndhf Sequences1
American Journal of Botany 89(9): 1531±1546. 2002. URTICALEAN ROSIDS: CIRCUMSCRIPTION, ROSID ANCESTRY, AND PHYLOGENETICS BASED ON RBCL, TRNL-F, AND NDHF SEQUENCES1 KENNETH J. SYTSMA,2,9 JEFFERY MORAWETZ,2,4 J. CHRIS PIRES,2,5 MOLLY NEPOKROEFF,2,6 ELENA CONTI,2,7 MICHELLE ZJHRA,2,8 JOCELYN C. HALL,2 AND MARK W. C HASE3 2Department of Botany, University of Wisconsin, Madison, Wisconsin 53706 USA, and 3Molecular Systematics Section, Royal Botanic Gardens, Kew, UK To address the composition of the urticalean rosids, the relationships of the component families (maximally Cannabaceae, Cecro- piaceae, Celtidaceae, Moraceae, Ulmaceae, and Urticaceae) and analyze evolution of morphological characters, we analyzed sequence variation for a large sampling of these families and various rosid outgroups using rbcL, trnL-F, and ndhF plastid regions. Urticalean rosids are derived out of a lineage including Barbeyaceae, Dirachmaceae, Elaeagnaceae, and Rhamnaceae, with Rosaceae less closely related; thus, they are imbedded within Rosales. Ulmaceae are the sister to all remaining families. Cannabaceae are derived out of a subclade of Celtidaceae; this expanded family should be called Cannabaceae. Cecropiaceae are derived within Urticaceae and are polyphyletic with Poikilospermum derived elsewhere within Urticaceae; this expanded family should be called Urticaceae. Monophy- letic Moraceae are sister to this expanded Urticaceae. Support for these relationships comes from a number of morphological characters (¯oral sexuality, presence or absence of hypanthium, stamen type and dehiscence, pollen pore number, ovule position, and embryo alignment) and chromosome numbers. Most fruit types, in terms of ecological dispersal, are derived independently multiple times and are strongly correlated with habitat. -

What Is a Tree in the Mediterranean Basin Hotspot? a Critical Analysis
Médail et al. Forest Ecosystems (2019) 6:17 https://doi.org/10.1186/s40663-019-0170-6 RESEARCH Open Access What is a tree in the Mediterranean Basin hotspot? A critical analysis Frédéric Médail1* , Anne-Christine Monnet1, Daniel Pavon1, Toni Nikolic2, Panayotis Dimopoulos3, Gianluigi Bacchetta4, Juan Arroyo5, Zoltán Barina6, Marwan Cheikh Albassatneh7, Gianniantonio Domina8, Bruno Fady9, Vlado Matevski10, Stephen Mifsud11 and Agathe Leriche1 Abstract Background: Tree species represent 20% of the vascular plant species worldwide and they play a crucial role in the global functioning of the biosphere. The Mediterranean Basin is one of the 36 world biodiversity hotspots, and it is estimated that forests covered 82% of the landscape before the first human impacts, thousands of years ago. However, the spatial distribution of the Mediterranean biodiversity is still imperfectly known, and a focus on tree species constitutes a key issue for understanding forest functioning and develop conservation strategies. Methods: We provide the first comprehensive checklist of all native tree taxa (species and subspecies) present in the Mediterranean-European region (from Portugal to Cyprus). We identified some cases of woody species difficult to categorize as trees that we further called “cryptic trees”. We collected the occurrences of tree taxa by “administrative regions”, i.e. country or large island, and by biogeographical provinces. We studied the species-area relationship, and evaluated the conservation issues for threatened taxa following IUCN criteria. Results: We identified 245 tree taxa that included 210 species and 35 subspecies, belonging to 33 families and 64 genera. It included 46 endemic tree taxa (30 species and 16 subspecies), mainly distributed within a single biogeographical unit. -

Glossy Buckthorn Rhamnusoriental Frangula Bittersweet / Frangula Alnus Control Guidelines Fact Sheet
Glossy buckthorn Oriental bittersweet Rhamnus frangula / Frangula alnus Control Guidelines Fact Sheet NH Department of Agriculture, Markets & Food, Division of Plant Industry, 29 Hazen Dr, Concord, NH 03301 (603) 271-3488 Common Name: Glossy buckthorn Latin Name: Rhamnus frangula / Frangula alnus New Hampshire Invasive Species Status: Prohibited (Agr 3800) Native to: Japan leaves (spring) Glossy buckthorn invasion Sapling (summer) Flowers (spring) Roadside invasion of saplings Fleshy fruits (fall) Gray bark w/ lenticels (Summer) Emodin in berries - effects to birds Fall foliage (Autumn) Description: Deciduous shrub or small tree measuring 20' by 15'. Bark: Grayish to brown with raised lenticels. Stems: Cinnamon colored with light gray lenticels. Leaves: Alternate, simple and broadly ovate. Flowers: Inconspicuous, 4- petaled, greenish-yellow, mid-May. Fruit: Fleshy, 1/4” diameter turning black in the fall. Zone: 3-7. Habitat: Adapts to most conditions including pH, heavy shade to full sun. Spread: Seeds are bird dispersed. Comments: Highly Aggressive, fast growing, outcompetes native species. Controls: Remove seedlings and saplings by hand. Larger trees can be cut or plants can be treated with an herbicide. General Considerations Glossy buckthorn can either grow as a multi-stemmed shrub or single-stemmed tree up to 23’ (7 m) tall. Leaves are deciduous, simple, and generally arranged alternately. Leaves are dark-green and glossy above while dull-green below. The leaf margins are smooth/entire and tend to be slightly wavy. Flowers are small, about ¼” and somewhat inconspicuous forming in May to June. They develop and in small clusters of 2-8. Fruits form in mid to late summer and contain 2-3 seeds per berry. -

Biology of a Rust Fungus Infecting Rhamnus Frangula and Phalaris Arundinacea
Biology of a rust fungus infecting Rhamnus frangula and Phalaris arundinacea Yue Jin USDA-ARS Cereal Disease Laboratory University of Minnesota St. Paul, MN Reed canarygrass (Phalaris arundinacea) Nature Center, Roseville, MN Reed canarygrass (Phalaris arundinacea) and glossy buckthorn (Rhamnus frangula) From “Flora of Wisconsin” Ranked Order of Terrestrial Invasive Plants That Threaten MN -Minnesota Terrestrial Invasive Plants and Pests Center Puccinia coronata var. hordei Jin & Steff. ✧ Unique spore morphology: ✧ Cycles between Rhamnus cathartica and grasses in Triticeae: o Hordeum spp. o Secale spp. o Triticum spp. o Elymus spp. ✧ Other accessory hosts: o Bromus tectorum o Poa spp. o Phalaris arundinacea Rust infection on Rhamnus frangula Central Park Nature Center, Roseville, MN June 2017 Heavy infections on Rhamnus frangula, but not on Rh. cathartica Uredinia formed on Phalaris arundinacea soon after mature aecia released aeiospores from infected Rhamnus frangula Life cycle of Puccinia coronata from Nazaredno et al. 2018, Molecular Plant Path. 19:1047 Puccinia coronata: a species complex Forms Telial host Aecial host (var., f. sp.) (primanry host) (alternate host) avenae Oat, grasses in Avenaceae Rhamnus cathartica lolii Lolium spp. Rh. cathartica festucae Fescuta spp. Rh. cathartica hoci Hocus spp. Rh. cathartica agronstis Agrostis alba Rh. cathartica hordei barley, rye, grasses in triticeae Rh. cathartica bromi Bromus inermis Rh. cathartica calamagrostis Calamagrostis canadensis Rh. alnifolia ? Phalaris arundinacea Rh. frangula Pathogenicity test on cereal crop species using aeciospores from Rhamnus frangula Cereal species Genotypes Response Oats 55 Immune Barley 52 Immune Wheat 40 Immune Rye 6 Immune * Conclusion: not a pathogen of cereal crops Pathogenicity test on grasses Grass species Genotypes Response Phalaris arundinacea 12 Susceptible Ph. -

Seed Dormancy and Consequences for Direct Tree Seeding H
Seed dormancy and consequences for direct tree seeding H. Frochot, Philippe Balandier, A. Sourisseau To cite this version: H. Frochot, Philippe Balandier, A. Sourisseau. Seed dormancy and consequences for direct tree seed- ing. Final COST E47 conference Forest vegetation management towards environmental sustainability, May 2009, Vejle, Denmark. p. 43 - p. 45. hal-00468732 HAL Id: hal-00468732 https://hal.archives-ouvertes.fr/hal-00468732 Submitted on 31 Mar 2010 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Forest Vegetation Management – towards environmental sustainability, N.S. Bentsen (Ed.), Proccedings from the final COST E47 Conference, Vejle, Denmark, 2009/05/5-7, Forest and Landscape Working Papers n° 35-2009, 43-45. Seed dormancy and consequences for direct tree seeding Henri Frochot1), Philippe Balandier2,3), Agnès Sourisseau4) 1) INRA, UMR1092 LERFOB, F-54280 Champenoux, France, [email protected]; [email protected] 2) Cemagref, UR EFNO, Nogent sur Vernisson, France 3) INRA, UMR547 PIAF, Clermont-Ferrand, France 4) Independent Landscaper, Paris, France Key words Afforestation, direct seeding, dormancy, seed, tree Introduction Whereas direct tree seeding was probably used extensively in France in the past, it is currently only employed for the reforestation of Pinus pinaster and some species with big seeds such as oak. -

Frangula Paruensis, a New Name for Rhamnus Longipes Steyermark (Rhamnaceae)
FRANGULA PARUENSIS, A NEW NAME FOR RHAMNUS LONGIPES STEYERMARK (RHAMNACEAE) GERARDO A. AYMARD C.1, 2 Abstract. The new name Frangula paruensis (Rhamnaceae) is proposed to replace the illegitimate homonym Rhamnus longipes Steyermark (1988). Chorological, taxonomic, biogeographical, and habitat notes about this taxon also are provided. Resumen. Se propone Frangula paruensis (Rhamnaceae) como un nuevo nombre para reemplazar el homónimo ilegítimo Rhamnus longipes Steyermark (1988). Se incluye información corológica, taxonómica, biogeográfica, y de hábitats acerca de la especie. Keywords: Frangula, Rhamnus, Rhamnaceae, Parú Massif, Tepuis flora, Venezuela Rhamnus L. and Frangula Miller (Rhamnaceae) have Frangula paruensis is a shrub, ca. 2 m tall, with leaves ca. 150 and ca. 50 species, respectively (Pool, 2013, 2015). ovate, or oblong-ovate, margin subrevolute, repand- These taxa are widely distributed around the world but are crenulate, a slightly elevated tertiary venation on the lower absent in Madagascar, Australia, and Polynesia (Medan and surface, and mature fruiting peduncle and pedicels 1–1.5 Schirarend, 2004). According to Grubov (1949), Kartesz cm long, and fruiting calyx lobes triangular-lanceolate and Gandhi (1994), Bolmgren and Oxelman (2004), and (two main features to separate Frangula from Rhamnus). Pool (2013) the recognition of Frangula is well supported. This species is endemic to the open, rocky savannas On the basis of historical and recent molecular work the on tepui slopes and summits at ca. 2000 m (Steyermark genus is characterized by several remarkable features. Pool and Berry, 2004). This Venezuelan taxon was described (2013: 448, table 1) summarized 11 features to separate the as Rhamnus longipes by Steyermark (1988), without two genera. -

Rhamnaceae) Jürgen Kellermanna,B
Swainsona 33: 43–50 (2020) © 2020 Board of the Botanic Gardens & State Herbarium (Adelaide, South Australia) Nomenclatural notes and typifications in Australian species of Paliureae (Rhamnaceae) Jürgen Kellermanna,b a State Herbarium of South Australia, GPO Box 1047, Adelaide, South Australia 5001 Email: [email protected] b The University of Adelaide, School of Biological Sciences, Adelaide, South Australia 5005 Abstract: The nomenclature of the four species of Ziziphus Mill. and the one species of Hovenia Thunb. occurring in Australia is reviewed, including the role of the Hermann Herbarium for the typification of Z. oenopolia (L.) Mill. and Z. mauritiana Lam. Lectotypes are chosen for Z. quadrilocularis F.Muell. and Z. timoriensis DC. A key to species is provided, as well as illustrations for Z. oenopolia, Z. quadrilocularis and H. dulcis Thunb. Keywords: Nomenclature, typification, Hovenia, Ziziphus, Rhamnaceae, Paliureae, Paul Hermann, Carolus Linnaeus, Henry Trimen, Australia Introduction last worldwide overview of the genus was published by Suessenguth (1953). Since then, only regional Rhamnaceae tribe Paliureae Reissek ex Endl. was treatments and revisions have been published, most reinstated by Richardson et al. (2000b), after the first notably by Johnston (1963, 1964, 1972), Bhandari & molecular analysis of the family (Richardson et al. Bhansali (2000), Chen & Schirarend (2007) and Cahen 2000a). It consists of three genera, Hovenia Thunb., et al. (in press). For Australia, the genus as a whole was Paliurus Tourn. ex Mill. and Ziziphus Mill., which last reviewed by Bentham (1863), with subsequent until then were assigned to the tribes Rhamneae regional treatments by Wheeler (1992) and Rye (1997) Horan. -

Phylogenetic Analysis of Vitaceae Based on Plastid Sequence Data
PHYLOGENETIC ANALYSIS OF VITACEAE BASED ON PLASTID SEQUENCE DATA by PAUL NAUDE Dissertation submitted in fulfilment of the requirements for the degree MAGISTER SCIENTAE in BOTANY in the FACULTY OF SCIENCE at the UNIVERSITY OF JOHANNESBURG SUPERVISOR: DR. M. VAN DER BANK December 2005 I declare that this dissertation has been composed by myself and the work contained within, unless otherwise stated, is my own Paul Naude (December 2005) TABLE OF CONTENTS Table of Contents Abstract iii Index of Figures iv Index of Tables vii Author Abbreviations viii Acknowledgements ix CHAPTER 1 GENERAL INTRODUCTION 1 1.1 Vitaceae 1 1.2 Genera of Vitaceae 6 1.2.1 Vitis 6 1.2.2 Cayratia 7 1.2.3 Cissus 8 1.2.4 Cyphostemma 9 1.2.5 Clematocissus 9 1.2.6 Ampelopsis 10 1.2.7 Ampelocissus 11 1.2.8 Parthenocissus 11 1.2.9 Rhoicissus 12 1.2.10 Tetrastigma 13 1.3 The genus Leea 13 1.4 Previous taxonomic studies on Vitaceae 14 1.5 Main objectives 18 CHAPTER 2 MATERIALS AND METHODS 21 2.1 DNA extraction and purification 21 2.2 Primer trail 21 2.3 PCR amplification 21 2.4 Cycle sequencing 22 2.5 Sequence alignment 22 2.6 Sequencing analysis 23 TABLE OF CONTENTS CHAPTER 3 RESULTS 32 3.1 Results from primer trail 32 3.2 Statistical results 32 3.3 Plastid region results 34 3.3.1 rpL 16 34 3.3.2 accD-psa1 34 3.3.3 rbcL 34 3.3.4 trnL-F 34 3.3.5 Combined data 34 CHAPTER 4 DISCUSSION AND CONCLUSIONS 42 4.1 Molecular evolution 42 4.2 Morphological characters 42 4.3 Previous taxonomic studies 45 4.4 Conclusions 46 CHAPTER 5 REFERENCES 48 APPENDIX STATISTICAL ANALYSIS OF DATA 59 ii ABSTRACT Five plastid regions as source for phylogenetic information were used to investigate the relationships among ten genera of Vitaceae. -

Shilin Yang Doctor of Philosophy
PHYTOCHEMICAL STUDIES OF ARTEMISIA ANNUA L. THESIS Presented by SHILIN YANG For the Degree of DOCTOR OF PHILOSOPHY of the UNIVERSITY OF LONDON DEPARTMENT OF PHARMACOGNOSY THE SCHOOL OF PHARMACY THE UNIVERSITY OF LONDON BRUNSWICK SQUARE, LONDON WC1N 1AX ProQuest Number: U063742 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest U063742 Published by ProQuest LLC(2017). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 ACKNOWLEDGEMENT I wish to express my sincere gratitude to Professor J.D. Phillipson and Dr. M.J.O’Neill for their supervision throughout the course of studies. I would especially like to thank Dr. M.F.Roberts for her great help. I like to thank Dr. K.C.S.C.Liu and B.C.Homeyer for their great help. My sincere thanks to Mrs.J.B.Hallsworth for her help. I am very grateful to the staff of the MS Spectroscopy Unit and NMR Unit of the School of Pharmacy, and the staff of the NMR Unit, King’s College, University of London, for running the MS and NMR spectra. -

Coastal Moonah Woodland in Victoria
A field guide to Coastal Moonah Woodland in Victoria A Victorian Government A Victorianinitiative Government initiative A field guide to Coastal Moonah Woodland in Victoria By Claire Moxham, Vivienne Turner, Gidja Walker and Imelda Douglas ISBN:978-1-74242-642-6 (print) ISBN: 978-1-74242-642-3 (on-line) © The State of Victoria, Department of Sustainability and Environment, 2010 This publication is copyright. Apart from any fair dealing for private study, research, criticism or review allowed under the Copyright Act 1968, no part of this publication may be reproduced, stored in a retrieval system or transmitted in any forms or by any means, electronic, photocopying or other, without the prior permission of the copyright holder. Published by the Victorian Government Department of Sustainability and Environment Melbourne, October 2010 Disclaimer: This publication may be of assistance to you but the State of Victoria and its employees do not guarantee that the publication is without flaw of any kind or is wholly appropriate for your particular purposes and therefore disclaims all liability for any error, loss or other consequence, which may arise from you relying on any information in this publication. This publication may be cited as: Citation: Moxham C., Turner V., Walker G. and Douglas I. (2010) A field guide to Coastal Moonah Woodland in Victoria. Arthur Rylah Institute for Environmental Research, Department of Sustainability and Environment, Melbourne. Front cover photo: Moonah (Melaleuca lanceolata subsp. lanceolata) by Claire Moxham Purpose This field guide provides information on the identification, ecology and management of Coastal Moonah Woodland (CMW) for use by land managers and naturalists. -

Ceanothus Crassifolius Torrey NRCS CODE: Family: Rhamnaceae (CECR) Order: Rhamnales Subclass: Rosidae Class: Magnoliopsida
I. SPECIES Ceanothus crassifolius Torrey NRCS CODE: Family: Rhamnaceae (CECR) Order: Rhamnales Subclass: Rosidae Class: Magnoliopsida Lower right: Ripening fruits, two already dehisced. Lower center: Longitudinal channeling in stems of old specimen, typical of obligate seeding Ceanothus (>25 yr since last fire). Note dark hypanthium in center of white flowers. Photos by A. Montalvo. A. Subspecific taxa 1. C. crassifolius Torr. var. crassifolius 2. C. crassifolius Torr. var. planus Abrams (there is no NRCS code for this taxon) B. Synonyms 1. C. verrucosus Nuttal var. crassifolius K. Brandegee (Munz & Keck 1968; Burge et al. 2013) 2. C. crassifolius (in part, USDA PLANTS 2019) C. Common name 1. hoaryleaf ceanothus, sometimes called thickleaf ceanothus or thickleaf wild lilac (Painter 2016) 2. same as above; flat-leaf hoary ceanothus and flat-leaf snowball ceanothus are applied to other taxa (Painter 2016) D. Taxonomic relationships Ceanothus is a diverse genus with over 50 taxa that cluster in to two subgenera. C. crassifolius has long been recognized as part of the Cerastes group of Ceanothus based on morphology, life-history, and crossing studies (McMinn 1939a, Nobs 1963). In phylogenetic analyses based on RNA and chloroplast DNA, Hardig et al. (2000) found C. crassifolius clustered into the Cerastes group and in each analysis shared a clade with C. ophiochilus. In molecular and morphological analyses, Burge et al. (2011) also found C. crassifolius clustered into Cerastes. Cerastes included over 20 taxa and numerous subtaxa in both studies. Eight Cerastes taxa occur in southern California (see I. E. Related taxa in region). E. Related taxa in region In southern California, the related Cerastes taxa include: C. -
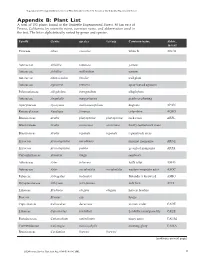
Vegetation and Ecological Characterisitics of Mixed-Conifer
Vegetation and Ecological Charactistics of Mixed-Conifer and Red Fir Forests at the Teakettle Experimental Forest Vegetation and Ecological Characteristics of Mixed-Conifer and Red Fir Forests at the Teakettle Experimental Forest Appendix B: Plant List A total of 152 plants found at the Teakettle Experimental Forest, 80 km east of Fresno, California, by scientific name, common name, and abbreviation used in the text. The list is alphabetically sorted by genus and species. Family Genus species var/ssp Common name Abbre. in text Pinaceae Abies concolor white fir ABCO Pinaceae Abies magnifica red fir ABMA Asteraceae Achillea lanulosa yarrow Asteraceae Achillea millefolium yarrow Asteraceae Adenocaulon bicolor trail plant Asteraceae Agroseris retrorsa spear-leaved agoseris Polemoniaceae Allophylum intregifolium allophylum Asteraceae Anaphalis margaritacea pearly everlasting Apocynaceae Apocynum androsaemifolium dogbane APAN Ranunculaceae Aquilegia formosa columbine AQFO Brassicaceae Arabis platysperma platysperma rock cress ARPL Brassicaceae Arabis rectissima rectissima bristly-leaved rock cress Brassicaceae Arabis repanda repanda repand rock cress Ericaceae Arctostaphylus nevadensis pinemat manzanita ARNE Ericaceae Arctostaphylus patula greenleaf manzanita ARPA Caryophyliaceae Arenaria kingii sandwort Asteraceae Aster foliaceus leafy aster ASFO Asteraceae Aster occidentalis occidentalis western mountain aster ASOC Fabaceae Astragalus bolanderi Bolander’s locoweed ASBO Dryopteridaceae Athryium felix-femina lady fern ATFI Liliaceae Brodiaea elegans elegans harvest brodeia Poaceae Bromus ssp. brome Cupressaceae Calocedrus decurrens incense cedar CADE Liliaceae Calochortus leichtlinii Leichtlin’s mariposa lily CALE Portulacaceae Calyptridium umbellatum pussy paws CAUM Convuvulaceae Calystegia malacophylla morning glory CAMA Brassicaceae Cardamine breweri breweri (continues on next page) 46 USDA Forest Service Gen.Tech. Rep. PSW-GTR-186. 2002. USDA Forest Service Gen.Tech. Rep.