Summary of Safety and Effectiveness Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
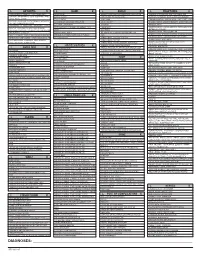
ICD-10 Diagnoses on Router
L ARTHRITIS R L HAND R L ANKLE R L FRACTURES R OSTEOARTHRITIS: PRIMARY, 2°, POST TRAUMA, POST _____ CONTUSION ACHILLES TEN DYSFUNCTION/TENDINITIS/RUPTURE FLXR TEN CLAVICLE: STERNAL END, SHAFT, ACROMIAL END CRYSTALLINE ARTHRITIS: GOUT: IDIOPATHIC, LEAD, CRUSH INJURY AMPUTATION TRAUMATIC LEVEL SCAPULA: ACROMION, BODY, CORACOID, GLENOID DRUG, RENAL, OTHER DUPUYTREN’S CONTUSION PROXIMAL HUMERUS: SURGICAL NECK 2 PART 3 PART 4 PART CRYSTALLINE ARTHRITIS: PSEUDOGOUT: HYDROXY LACERATION: DESCRIBE STRUCTURE CRUSH INJURY PROXIMAL HUMERUS: GREATER TUBEROSITY, LESSER TUBEROSITY DEP DIS, CHONDROCALCINOSIS LIGAMENT DISORDERS EFFUSION HUMERAL SHAFT INFLAMMATORY: RA: SEROPOSITIVE, SERONEGATIVE, JUVENILE OSTEOARTHRITIS PRIMARY/SECONDARY TYPE _____ LOOSE BODY HUMERUS DISTAL: SUPRACONDYLAR INTERCONDYLAR REACTIVE: SECONDARY TO: INFECTION ELSEWHERE, EXTENSION OR NONE INTESTINAL BYPASS, POST DYSENTERIC, POST IMMUNIZATION PAIN OCD TALUS HUMERUS DISTAL: TRANSCONDYLAR NEUROPATHIC CHARCOT SPRAIN HAND: JOINT? OSTEOARTHRITIS PRIMARY/SECONDARY TYPE _____ HUMERUS DISTAL: EPICONDYLE LATERAL OR MEDIAL AVULSION INFECT: PYOGENIC: STAPH, STREP, PNEUMO, OTHER BACT TENDON RUPTURES: EXTENSOR OR FLEXOR PAIN HUMERUS DISTAL: CONDYLE MEDIAL OR LATERAL INFECTIOUS: NONPYOGENIC: LYME, GONOCOCCAL, TB TENOSYNOVITIS SPRAIN, ANKLE, CALCANEOFIBULAR ELBOW: RADIUS: HEAD NECK OSTEONECROSIS: IDIOPATHIC, DRUG INDUCED, SPRAIN, ANKLE, DELTOID POST TRAUMATIC, OTHER CAUSE SPRAIN, ANKLE, TIB-FIB LIGAMENT (HIGH ANKLE) ELBOW: OLECRANON WITH OR WITHOUT INTRA ARTICULAR EXTENSION SUBLUXATION OF ANKLE, -

First Metatarsophalangeal Joint Replacement with Total Arthroplasty in the Surgical Treatment of the Hallux Rigidus R
Acta Biomed 2014; Vol. 85, Supplement 2: 113-117 © Mattioli 1885 Original article First metatarsophalangeal joint replacement with total arthroplasty in the surgical treatment of the hallux rigidus R. Valentini, G. De Fabrizio, G. Piovan Clinica Ortopedica e Traumatologica, Università degli Studi di Trieste, Azienda Ospedaliero-Universitaria “Ospedali Riuniti” di Trieste Abstract. The hallux rigidus, especially in advanced stage, has always been a challenge as regards the surgical treatment. Over the years there have been various surgical techniques proposed with the aim of relieving pain, correcting deformity and maintain a certain degree of movement. For some years we have addressed the prob- lem with the replacement metatarsophalangeal joint arthroplasty with Reflexion system. As far as our experi- ence we have operated and monitored 25 patients (18 females and 7 males) of mean age 58.1 years, operated with this technique from June 2008 to June 2011. It reached an average ROM of 72° (extension and flexion 45° and 27°) with a good functional recovery in 8 patients, and this articulation was good (50° - 40°) in 12 patients and moderate in 5 with a articular range from 40°- 30°. The clinical results, according to our experience, appear to be favorable, as even patient satisfaction is complete. (www.actabiomedica.it) Key words: hallux rigidus, metatarsophalangeal, arthroprosthesis Introduction The pathology of stiff big toe has ranked about Regnauld classification (5) in three stages, so that the Degenerative disease of the first metatarsal- I stage is characterized by wear of the joint with mini- phalangeal articulation, the so-called “Hallux rigi- mal osteophytes reaction, the II stage is reached when dus”, especially in advanced phase, has always been the joint line is further reduced, the articular surfaces a sort of challenge as a surgical treatment. -

Bilateral Hallux Varus Deformity Correction with a Suture Button Construct
A Case Report & Literature Review Bilateral Hallux Varus Deformity Correction With a Suture Button Construct Andrew R. Hsu, MD, Christopher E. Gross, MD, and Johnny L. Lin, MD common surgery for hallux varus correction is transfer of the Abstract extensor hallucis longus partially or completely under the deep Hallux varus deformity typically results from transverse intermetatarsal ligament to the lateral aspect base soft-tissue overcorrection at the metatarsopha- of the proximal phalanx.10,11 However, complications include langeal joint during surgery for hallux valgus. weakened extension and the inability to fix an overcorrected There are several soft-tissue procedures avail- intermetatarsal angle. Alternatively, the abductor hallucis can able for flexible hallux varus deformity includ- be tenotomized or transferred to the base of the proximal ing transfer of the extensor hallucis longus or phalanx to reconstruct the lateral capsular ligaments.8,9 The abductor hallucis. To our knowledge, there have lateral capsular ligaments can be reinforced or reconstructed not been any previous reports in the literature of with fascia lata or soft-tissue anchors, but these procedures rely bilateral hallux varus deformities in the setting of on adequate healthy tissue remaining around the MTP joint.9,12 potential pregnancy-related ligamentous laxity The Mini TightRope (Arthrex Inc, Naples, Florida) is an im- combined with iatrogenic injury. We present the planted suture endobutton device that has previously been used 13 case of an isolated bilateral hallux varus defor- for hallux valgus repair. The suture button technique has also mity occurring after pregnancy and prior bunion been used to recreate ligaments and tendons in syndesmotic 14,15 14 surgery. -

Biomechanics of Nerve and Muscle
The Biomechanics of the Human Lower Extremity DR.AYESH BASHARAT BSPT, PP.DPT. M.Phil (Gold-medalist) Hip joint One of the largest and most stable joint: The hip joint Rigid ball-and-socket configuration (Intrinsic stability) The femoral head Femoral head : convex component Two-third of a sphere, Cover with cartilage Rydell (1965) suggested : most load----- superior quadrant Femur Long, strong & most weight bearing bone. But most weakest structure of it is its neck. During walk in single leg support move medially to support C0G. This results in the leg being shortened on non-weight bearing side. Fracture of femur-neck common as bone tissue in the neck of the femur is softer than normal. Acetabulum Concave component of ball and socket joint Facing obliquely forward, outward and downward Covered with articular cartilage Provide static stability Labrum: a flat rim of fibro cartilage Acetabulum Also contain Transverse acetabular ligament provide stability Ligaments and Bursae •Iliofemoral ligament: Y shaped extremely strong= anterior stability •Pubofemoral ligament: anterior stability • Ischiofemoral ligament: posterior stability • Ligamentum teressupplies a direct attachment from rim of acetabulum to head of femur Iliopsoas burs b/w illiopsoas & capsule Trochantric bursitis The femoral neck Frontal plane (the neck-to-shaft angle/ angle of inclination), Transverse plane (the angle of anteversion) Neck-to- shaft angle : 125º, vary from 110º to 135-140º Effect : lever arms Angle of anteversion :12º Effect : during gait >12º :internal rotation <12º :external rotation aa The anteverted femur effect the biomechanics of not only hip joint but also disturbed the knee and ankle joint normal mechanics during different physical activities a Structure of the Hip Sacrum Ilium Acetabulum Femoral head Pubis Ischium Femur The pelvic girdle includes the two ilia and the sacrum,. -

Understanding the First
CME / ORTHOTICS & BIOMECHANICS Goals and Objectives After reading this CME the practitioner will be able to: 1) Understand normal and abnormal function of the first ray with special emphasis on its integral role in medial longitudinal arch function and hypermobility. Understanding 2) Acquire knowledge of the various etiologic factors that result in first ray hypermobility. the First Ray 109 3) Appreciate its normal and abnormal motion along Here’s a review of its normal with its attendant bio and and abnormal function, identification, pathomechanics. and clinical significance. 4) Become familiar with vari- ous methods to subjectively and BY JOSEPH C D’AMICO, DPM objectively identify its presence. Welcome to Podiatry Management’s CME Instructional program. Our journal has been approved as a sponsor of Con- tinuing Medical Education by the Council on Podiatric Medical Education. You may enroll: 1) on a per issue basis (at $26.00 per topic) or 2) per year, for the special rate of $210 (you save $50). You may submit the answer sheet, along with the other information requested, via mail, fax, or phone. You can also take this and other exams on the Internet at www.podiatrym.com/cme. If you correctly answer seventy (70%) of the questions correctly, you will receive a certificate attesting to your earned credits. You will also receive a record of any incorrectly answered questions. If you score less than 70%, you can retake the test at no additional cost. A list of states currently honoring CPME approved credits is listed on pg. 144. Other than those entities currently accepting CPME-approved credit, Podiatry Management cannot guarantee that these CME credits will be acceptable by any state licensing agency, hospital, managed care organization or other entity. -

Foot Orthotics and Other Podiatric Appliances
MEDICAL POLICY POLICY TITLE FOOT ORTHOTICS AND OTHER PODIATRIC APPLIANCES POLICY NUMBER MP 6.028 Original Issue Date (Created): 7/1/2002 Most Recent Review Date (Revised): 3/19/2021 Effective Date: 8/1/2021 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY APPENDIX I. POLICY Orthopedic shoes and other supportive devices of the feet are considered medically necessary ONLY when they are an integral part of a leg brace. These shoes and devices are described as Oxford shoes or other shoes, e.g. high top, depth inlay or custom for non- diabetics, heel replacements, sole replacements, and shoe transfers. Inserts and other shoe modifications are covered if they are on a shoe that is an integral part of a covered brace and if they are medically necessary for the proper functioning of the brace. Foot orthotics other than those that are an integral part of a brace may be considered medically necessary only when they are a benefit of a member’s contract, to meet specific needs of the patient, and prescribed by a physician for the below criteria: For Adults and Children [Any ONE Condition]: Chronic plantar fasciitis Calcaneal bursitis (chronic only) Calcaneal spurs (heel spurs) Chronic ankle instability Inflammatory conditions (i.e., sesamoiditis; submetatarsal bursitis; synovitis; tenosynovitis; synovial cyst; osteomyelitis; rheumatoid disease; and osteoarthritis) Medial osteoarthritis of the knee (lateral wedge insoles) Musculoskeletal/arthropathic -

Hallux Valgus CONTRIBUTORS Matthew D
FASXXX10.1177/1938640016640403Foot & Ankle SpecialistFoot & Ankle Specialist 640403research-article2016 vol. 9 / no. 2 Foot & Ankle Specialist 159 〈 Roundtable Discussion〉 Hallux Valgus CONTRIBUTORS Matthew D. Sorensen, DPM Weil Foot and Ankle Institute Are We Really Getting It Correct? Chicago, IL Truitt M. Cooper, MD Assistant Professor o say that the topic of bunions understand that each bunion deformity is Department of Orthopedics comes into play in all of our different with its own set of University of Virginia T practices would be stating the idiosyncrasies. I think the subsets are Charlottesville, VA obvious. Whether you deal with them few and therefore enable us to make often and find enjoyment in the cases, or educated decisions when it comes to Paul Dayton, DPM you see them rarely and hide in your picking the appropriate procedure for Unity Point Clinic office when a patient shows up with a any given bunion deformity. I would Fort Dodge, IA bunion complaint, they affect our practice. strongly submit that a “one trick pony” Assistant Professor My major frustration with bunions has approach to correcting a bunion is Des Moines University CPMS often been the unpredictability of the probably not appropriate. Des Moines, IA outcomes. I felt this was an area I was not Cooper: Not really. When we work with ROUNDTABLE MODERATOR happy about in my own practice. My the residents and try to help them get thought was, “Come on, I’m the foot and ready for the board exams, we make sure W. Bret Smith, DO, MS ankle guy in my practice, I should be they know how to measure intermetatarsal Director of Foot and Ankle Division knocking this out of the park.” (IM) angles and hallux valgus angles, as Moore Center for Orthopedics So I thought I would assemble a team of well as to look for congruency at the Columbia, SC innovative, forward-thinking surgeons to metatarsophalangeal (MTP) joint. -

Reverse Transfer of the Abductor Hallucis Tendon a Report of 7 Cases
Acta Orthop. Belg., 2008, 74, 227-234 ORIGINAL STUDY A new surgical procedure for iatrogenic hallux varus : Reverse transfer of the abductor hallucis tendon A report of 7 cases Thibaut LEEMRIJSE, Bao HOANG, Pierre MALDAGUE, Pierre-Louis DOCQUIER, Bernhard DEVOS BEVERNAGE From Saint-Luc University Hospital, Brussels, Belgium Iatrogenic hallux varus is a possible complication of INTRODUCTION hallux valgus surgery following Mc Bride or Scarf osteotomy, with or without Akin osteotomy of the Hallux varus deformity is a condition in which first phalanx. It may also occur following chevron the great toe is medially oriented on the first osteotomy or Keller’s procedure. One possibility for metatarsal head. It is often a triplane deformity in surgical revision of iatrogenic hallux varus is recon- which the medially deviated hallux is also ham- struction of the lateral stabilising soft-tissue compo- mered and supinated (7). nents of the first metatarsophalangeal joint. Until The condition can be congenital or acquired. now, only dynamic tendon transfers, possibly com- Several subtypes of acquired hallux varus can bined with interphalangeal fusion, have been be seen : iatrogenic, traumatic, following burn, in described. The aim of our study was to develop a systemic disorders (rheumatoid arthritis, psoriatic static, anatomic reconstruction procedure. arthritis), in Charcot-Marie-Tooth disease, in case A new surgical technique of ligamentoplasty using of avascular necrosis of the metatarsal head and in the abductor hallucis tendon is described. The new 2,3,17,23 method was applied in 7 feet (5 patients) with a mean poliomyelitis ( ). follow-up over two years. Hallux varus deformities were operated by transplantation of the abductor hallucis tendon. -

Orthotic Devices and Shoes – (0543)
Medical Coverage Policy Effective Date ............................................. 7/15/2021 Next Review Date ....................................... 8/15/2022 Coverage Policy Number .................................. 0543 Orthotic Devices and Shoes Table of Contents Related Coverage Resources Overview .............................................................. 1 Extracorporeal Shock Wave Therapy (ESWT) for Coverage Policy ................................................... 2 Musculoskeletal Conditions and Soft Tissue General Background ............................................ 8 Wounds Medicare Coverage Determinations .................. 20 Foot Care Services Coding/Billing Information .................................. 21 Lumbar Fusion for Spinal Instability and Degenerative Disc Conditions, including Sacroiliac Fusion References ........................................................ 47 Minimally Invasive Spine Surgery Procedures and Trigger Point Injections Percutaneous Vertebroplasty, Kyphoplasty and Sacroplasty Physical Therapy Plantar Fasciitis Treatments Prosthetic Devices Stretch Devices for Joint Stiffness and Contracture Subtalar Arthroereisis INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna Companies. Certain Cigna Companies and/or lines of business only provide utilization review services to clients and do not make coverage determinations. References to standard benefit plan language and coverage determinations do not apply to those clients. Coverage Policies -

OITE Foot and Ankle Review Anatomy and Biomechanics
OITE Foot and Ankle Review Anatomy and Biomechanics • Bones and Ligaments – The Ankle Joint • Includes the Tibia, talus and fibula • Joint is trapezoidal and wider anteriorly • Talus only tarsal bone without muscular or ligamentous insertions – Lateral Ankle Ligaments • Anterior Talofibular Ligament (ATFL) – Under strain in plantar flexion, inversion, and Internal rotation • Calcaneofibular Ligament (CFL) – Under strain in dorsiflexion and inversion • Posterior Talofibular Ligament (PTFL) Anatomy and Biomechanics • Deltoid Ligament – Triangular shaped ligament with the apex at the medial malleolus and extending to the calcaneus, talus, and navicular – Divided into: • Superficial component – Three parts: anterior to navicular, inferior to sustanaculum, and posterior to talar body • Deep component – Two bands from medial malleolus to talar body just inferior to the medial facet • Syndesmosis – Ligamentous structures • Anterior inferior tibiofibular ligament (AITFL) • Interosseous ligament • Posterior inferior tibiofibular ligament • Transverse tibiofibular ligament Anatomy and Biomechanics • Hindfoot and Midfoot – Subtalar Joint • Three Facets: Posterior (Largest), Middle (medial and rests on sustenacum tali), and anterior (continuous with talonavicular joint) • Transverse Tarsal Joint (Chopart Joint) – Talonavicular and Calcaneocuboid Joints – Talonavicular Joint supported by two ligaments (Spring Ligament): the superior medial calcaneonavicular ligament (SMCN) and the inferior calcaneonavicular (ICN) ligament • Most likely attenuated in -

Toes: Anatomy, Pathology and Common Surgical Procedures
Toes: Anatomy, Pathology and Common Surgical Procedures Adam Singer, MD1; Jason Bariteau, MD2; Yara Younan, MD1; Walter Carpenter, MD1 Jean Jose, MD3; Ty Subhawong, MD3; Monica Umpierrez, MD1 1 Emory University Hospital, Department of Radiology Section of Musculoskeletal Imaging, USA 2 Emory University Hospital, Department of Orthopedic Surgery, USA 3 University of Miami, Department of Radiology Section of Musculoskeletal Imaging, USA Learning Objectives 1. Osseous and soft tissue toe anatomy a. The great toe b. The lesser toes 2. Pathophysiology and clinical presentation of injury to great and lesser toes a. Hallux valgus, varus and rigidus b. Bunion and bunionette c. Hallux sesamoid complex injury and turf toe d. Hammer , claw and mallet toe e. Freiberg infraction f. Neuroma g. Benign masses a. Subungal exostosis b. Plantar fibroma 3. Commonly encountered surgical procedures Authors have no conflict of interest. Osseous and Soft Tissue Anatomy Overview Joint Muscle belly 2nd DIP Tendon 2nd PIP 1st IP Osseous structure Dorsal Interosseous Dorsal Interosseous Plantar Interosseous Medial Collateral Ligament Complex Medial Collateral Ligament Complex Lateral Collateral MS Ligament Complex Adductor Hallucis FDL LS FHL 1st MTP 1st MTP Lumbricals Abductor Hallucis FHB FHB Plantar FHB FHB Interosseous FDB FDB Osseous and Soft Tissue Anatomy: The Great Toe Joint Ligament EHL Tendon Proximal Intersesamoidal ligament Osseous structure 1st MT Proximal phalanx Hallux Sesamoid articular complex Adductor Hallucis EHL FHL Abductor Hallucis Distal Crista -

First Metatarsophalangeal Joint Fusion with Minimal Fixation
CHAPTER 36 FIRST METATARSOPHALANGEAL JOINT FUSION WITH MINIMAL FIXATION John V. Vanore, DPM First metatarsophalangeal (MTP) joint fusion has been an modern era of first MTP joint arthrodesis but interestingly he operative technique utilized as both primary treatment as also describes unintentional ankylosis in one of his patients well as a revisionary one for pathology involving the first that had undergone bilateral bunion surgery. 23 The patient MTP joint. 1-9 Many papers have attempted to investigate the had an infection and postoperative stiffness. The patient ideal combination of joint resection techniques and fixation preferred the result and he also felt that the foot that configurations to provide maximum stability to aid suffered the complication actually seem to function better. It arthrodesis. 10-21 Fixation techniques have spanned the is from these observations of McKeever and Glissan before entire gamut of alternatives from simple pin fixation to him that led to the orthopedic community to embrace first compression techniques with multiple cross screws to more MPJ arthrodesis in the mid-20th century. Crumble (1956) complex fixation constructs utilizing a plate and screws. and Stamm (1957) recommended alternative procedures to joint fusion unless presented with hallux rigidus and gross FIRST MTP JOINT ARTHRODESIS arthritic change to the joint. 22 Interestingly, some of the earliest techniques of First MTP joint arthrodesis may be recommended as a McKeever and his colleagues included attempts at rigid primary procedure or one of revision in cases of prior internal fixation utilizing metallic screws or external surgery. It is this latter group that this paper will address.