Orthotic Devices and Shoes – (0543)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Union County Veterans Bulletin February 2021
F E B R U A R Y 2 0 2 1 | I S S U E 4 THE VETERAN'S BULLETIN The Official Newsletter of the Union County Office of Veteran Services Welcome to The Veteran's Bulletin, the official newsletter of the Union County Office of Veteran Services. This publication is intended to keep our In this Issue: Veterans and their families informed about programs, services and events for Union County Veterans and Union County Veterans active military residents. Parking Placards - 1,2 LGBTQ Veteran News - 2 New VA Secretary -3 Veterans Programs - 3 Black History Month - 3 COVID-19 Vaccine Center opens in Union County - 4 Secondary Disability Claims for Veterans - 5 Veteran Resources and Directory Services - 6 County Designates New Parking Sites For Union County Veterans UNioN CouNty CommissioNer ChairmaN AlexaNder Mirabella joiNed officials from the UNioN CouNty Office of VeteraNs Services, aNd the Office of the UNioN CouNty Prosecutor oN November 10, 2020 at the Ash Brook Golf Course iN Scotch PlaiNs to iNtroduce New desigNated parkiNg spaces for VeteraNs at NiNe couNty facilities. The free parkiNg is available to aNy couNty VeteraN who applies for a parkiNg placard through the UNioN CouNty Office of VeteraNs Services. For more iNformatioN oN how to apply aNd receive the parkiNg placard, please read the article oN page 2. T H E V E T E R A N S B U L L E T I N P A G E 2 County Designates Parking Sites For Veterans IN hoNor of VeteraNs Day, the UNioN CouNty Board of CouNty CommissioNers approved New parkiNg spaces at NiNe couNty facilities for military veteraNs. -

Beyond Diversity and Inclusion: Understanding and Addressing Ableism, Heterosexism, and Transmisia in the Legal Profession: Comm
American Journal of Law & Medicine, 47 (2021): 76-87 © 2021 The Author(s) DOI: 10.1017/amj.2021.3 Beyond Diversity and Inclusion: Understanding and Addressing Ableism, Heterosexism, and Transmisia in the Legal Profession: Comment on Blanck, Hyseni, and Altunkol Wise’s National Study of the Legal Profession Shain A. M. Neumeier† and Lydia X. Z. Brown†† I. INTRODUCTION Far too many—if not most—of us in the legal profession who belong to both the disability and LGBTQþ communities have known informally, through our own experi- ences and those of others like us, that workplace bias and discrimination on the basis of disability, sexuality, and gender identity is still widespread. The new study by Blanck et al. on diversity and inclusion in the U.S. legal profession provides empirical proof of this phenomenon, which might otherwise be dismissed as being based on anecdotal evidence.1 Its findings lend credibility to our position that the legal profession must make systemic changes to address workplace ableism, heterosexism, and transmisia.2 They also suggest †Committee for Public Counsel Services, Mental Health Litigation Division. (The contents of this article are published in the author’s personal capacity.) ††Georgetown University, Disability Studies Program, [email protected]. With thanks to Sara M. Acevedo Espinal, Jennifer Scuro, and Jess L. Cowing for support. 1Peter Blanck, Fitore Hyseni & Fatma Artunkol Wise, Diversity and Inclusion in the American Legal Profession: Discrimination and Bias Reported by Lawyers with Disabilities and Lawyers Who Identify as LGBTQþ,47Am. J.L. & Med. 9, 9 (2021) [hereinafter Blanck, et al., Discrimination and Bias]. -

Guidelines for Inclusion: Ensuring Access to Education for All Acknowledgments
Guidelines for Inclusion: Ensuring Access to Education for All Acknowledgments In UNESCO’s efforts to assist countries in making National Plans for Education more inclusive, we recognised the lack of guidelines to assist in this important process. As such, the Inclusive Education Team, began an exercise to develop these much needed tools. The elaboration of this manual has been a learning experience in itself. A dialogue with stakeholders was initiated in the early stages of elaboration of this document. “Guidelines for Inclusion: Ensuring Access to Education for All”, therefore, is the result of constructive and valuable feedback as well as critical insight from the following individuals: Anupam Ahuja, Mel Ainscow, Alphonsine Bouya-Aka Blet, Marlene Cruz, Kenneth Eklindh, Windyz Ferreira, Richard Halperin, Henricus Heijnen, Ngo Thu Huong, Hassan Keynan, Sohae Lee, Chu Shiu-Kee, Ragnhild Meisfjord, Darlene Perner, Abby Riddell, Sheldon Shaeffer, Noala Skinner, Sandy Taut, Jill Van den Brule-Balescut, Roselyn Wabuge Mwangi, Jamie Williams, Siri Wormnæs and Penelope Price. Published in 2005 by the United Nations Educational, Scientifi c and Cultural Organization 7, place de Fontenoy, 75352 PARIS 07 SP Composed and printed in the workshops of UNESCO © UNESCO 2005 Printed in France (ED-2004/WS/39 cld 17402) Foreward his report has gone through an external and internal peer review process, which targeted a broad range of stakeholders including within the Education Sector at UNESCO headquarters and in the fi eld, Internal Oversight Service (IOS) and Bu- Treau of Strategic Planning (BSP). These guidelines were also piloted at a Regional Work- shop on Inclusive Education in Bangkok. -

Scaffolding Inquiry-Based Science and Chemistry Education in Inclusive Classrooms
In: New Developments in Science Education Research ISBN: 978-1-63463-914-9 Editor: Nathan L. Yates © 2015 Nova Science Publishers, Inc. Chapter 5 SCAFFOLDING INQUIRY-BASED SCIENCE AND CHEMISTRY EDUCATION IN INCLUSIVE CLASSROOMS Simone Abels University of Vienna, Austrian Educational Competence Centre Chemistry, Vienna, Austria ABSTRACT Since the ratification of the UN Convention on Rights of Persons with Disabilities inclusion is on the agenda. In science education inquiry-based learning is, among others, recommended to deal with the diversity of students in a classroom. However, teachers struggle with the implementation of inquiry-based science education and view heterogeneity rather as a burden than an asset. Therefore, this research project is dedicated to the teachers‘ scaffolding teaching science in inclusive classes. Specifically, within the framework of a qualitative case study, the focus lies upon inquiry-based learning environments with different levels of openness and structuring and how they can be orchestrated to deal with diverse learning needs of all students in one classroom. The research study is conducted in cooperation with an inclusive urban middle school (grade 5 to 8), where students with and without special educational needs learn together. Initially, the article introduces the inclusive school with its specific way and challenges – not only the science classes – as inclusion is holistic school development. Subsequently, two science formats are particularly focused and contrasted: guided inquiry-based chemistry lessons as well as the format Lernwerkstatt (German for workshop center), an open inquiry setting. The aim of the Lernwerkstatt is that students work self-dependently on their own scientific questions innervated by carefully arranged phenomena and materials. -
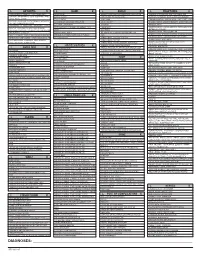
ICD-10 Diagnoses on Router
L ARTHRITIS R L HAND R L ANKLE R L FRACTURES R OSTEOARTHRITIS: PRIMARY, 2°, POST TRAUMA, POST _____ CONTUSION ACHILLES TEN DYSFUNCTION/TENDINITIS/RUPTURE FLXR TEN CLAVICLE: STERNAL END, SHAFT, ACROMIAL END CRYSTALLINE ARTHRITIS: GOUT: IDIOPATHIC, LEAD, CRUSH INJURY AMPUTATION TRAUMATIC LEVEL SCAPULA: ACROMION, BODY, CORACOID, GLENOID DRUG, RENAL, OTHER DUPUYTREN’S CONTUSION PROXIMAL HUMERUS: SURGICAL NECK 2 PART 3 PART 4 PART CRYSTALLINE ARTHRITIS: PSEUDOGOUT: HYDROXY LACERATION: DESCRIBE STRUCTURE CRUSH INJURY PROXIMAL HUMERUS: GREATER TUBEROSITY, LESSER TUBEROSITY DEP DIS, CHONDROCALCINOSIS LIGAMENT DISORDERS EFFUSION HUMERAL SHAFT INFLAMMATORY: RA: SEROPOSITIVE, SERONEGATIVE, JUVENILE OSTEOARTHRITIS PRIMARY/SECONDARY TYPE _____ LOOSE BODY HUMERUS DISTAL: SUPRACONDYLAR INTERCONDYLAR REACTIVE: SECONDARY TO: INFECTION ELSEWHERE, EXTENSION OR NONE INTESTINAL BYPASS, POST DYSENTERIC, POST IMMUNIZATION PAIN OCD TALUS HUMERUS DISTAL: TRANSCONDYLAR NEUROPATHIC CHARCOT SPRAIN HAND: JOINT? OSTEOARTHRITIS PRIMARY/SECONDARY TYPE _____ HUMERUS DISTAL: EPICONDYLE LATERAL OR MEDIAL AVULSION INFECT: PYOGENIC: STAPH, STREP, PNEUMO, OTHER BACT TENDON RUPTURES: EXTENSOR OR FLEXOR PAIN HUMERUS DISTAL: CONDYLE MEDIAL OR LATERAL INFECTIOUS: NONPYOGENIC: LYME, GONOCOCCAL, TB TENOSYNOVITIS SPRAIN, ANKLE, CALCANEOFIBULAR ELBOW: RADIUS: HEAD NECK OSTEONECROSIS: IDIOPATHIC, DRUG INDUCED, SPRAIN, ANKLE, DELTOID POST TRAUMATIC, OTHER CAUSE SPRAIN, ANKLE, TIB-FIB LIGAMENT (HIGH ANKLE) ELBOW: OLECRANON WITH OR WITHOUT INTRA ARTICULAR EXTENSION SUBLUXATION OF ANKLE, -

Assisted Living & Memory Care
Engaging Lifestyle Assisted Living & Memory Care We offer an extensive, wellness-focused activity program that is tailored to at Diakon Senior Living – Hagerstown individual needs, desires and abilities. From art, movies and music therapy to educational opportunities, card games and cooking instruction, we offer a lifestyle that is both Support. Activity. Comfort. Peace of Mind. fulfilling and rewarding. Our programming is infused with a range of activities designed to pique the interests of residents with varying backgrounds and life experiences. This is the Exceptional Dining Way to Live! Forget cooking! Instead, enjoy three delicious, chef-prepared meals per day (plus snacks) in a bright and welcoming dining room. Families are always welcome to join! Catering services are also available for private gatherings and special events. Community Amenities Call today to schedule your personal visit. Our beautiful setting is complemented by many amenities designed to make life more comfortable and fun. Residents enjoy a salon, 240.420.4133 | www.HagerstownSeniorLiving.org library, fitness center, heated pool and many other conveniences. Robinwood Campus 19800 Tranquility Circle | Hagerstown, MD 21742 Worship Services Ravenwood Campus 1183 Luther Drive | Hagerstown, MD 21740 Our two assisted living communities offer the perfect At Diakon Senior Living - Hagerstown, our residents Residents have the opportunity to attend solution for those who desire an active, fulfilling have everything they need to live fully – including religious services, representing a variety of lifestyle but can no longer live alone safely. We offer privacy, support and daily opportunities to be engaged faiths, right on campus. We also offer Bible personalized care plans that are based on unique needs and inspired – while their families enjoy the peace of study programs, and our staff chaplain is and care preferences. -

Prototype and Script.Aculo.Us You Never Knew Javascript Could Do This!
Prototype and script.aculo.us You Never Knew JavaScript Could Do This! Christophe Porteneuve The Pragmatic Bookshelf Raleigh, North Carolina Dallas, Texas Many of the designations used by manufacturers and sellers to distinguish their prod- ucts are claimed as trademarks. Where those designations appear in this book, and The Pragmatic Programmers, LLC was aware of a trademark claim, the designations have been printed in initial capital letters or in all capitals. The Pragmatic Starter Kit, The Pragmatic Programmer, Pragmatic Programming, Pragmatic Bookshelf and the linking g device are trademarks of The Pragmatic Programmers, LLC. Every precaution was taken in the preparation of this book. However, the publisher assumes no responsibility for errors or omissions, or for damages that may result from the use of information (including program listings) contained herein. Our Pragmatic courses, workshops, and other products can help you and your team create better software and have more fun. For more information, as well as the latest Pragmatic titles, please visit us at http://www.pragprog.com Copyright © 2007 The Pragmatic Programmers LLC. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmit- ted, in any form, or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior consent of the publisher. Printed in the United States of America. ISBN-10: 1-934356-01-8 ISBN-13: 978-1-934356-01-2 To Élodie, my love, ever supportive. You’re my true home. Contents Preface 13 1 Introduction 15 1.1 It’s About Time ........................ 15 1.2 What’s in This Book, and How Is It Organized? . -

Eliminating Ableism in Education
Eliminating Ableism in Education THOMAS HEHIR Harvard Graduate School of Education In this article, Thomas Hehir defines ableism as “the devaluation of disability” that “results in societal attitudes that uncritically assert that it is better for a child to walk than roll, speak than sign, read print than read Braille, spell independently than use a spell-check, and hang out with nondisabled kids as opposed to other dis- abled kids.” Hehir highlights ableist practices through a discussion of the history of and research pertaining to the education of deaf students, students who are blind or visually impaired, and students with learning disabilities, particularly dyslexia. He asserts that “the pervasiveness of...ableistassumptionsintheeducationof children with disabilities not only reinforces prevailing prejudices against disability but may very well contribute to low levels of educational attainment and employ- ment.” In conclusion, Hehir offers six detailed proposals for beginning to address and overturn ableist practices. Throughout this article, Hehir draws on his per- sonal experiences as former director of the U.S. Department of Education’s Office of Special Education Programs, Associate Superintendent for the Chicago Public Schools, and Director of Special Education in the Boston Public Schools. Ableist Assumptions When Joe Ford was born in 1983, it was clear to the doctors and to Joe’s mom Penny that he would likely have disabilities. What wasn’t clear to Penny at the time was that she was entering a new world, that of a parent of a child with disabilities, a world in which she would have to fight constantly for her child to have the most basic of rights, a world in which deeply held negative cul- tural assumptions concerning disability would influence every aspect of her son’s life. -

Inquiring Minds Want to Know: Inquiry-Based Learning in the General Education and Inclusion Science Classroom
129 Inquiring Minds Want to Know: Inquiry-Based Learning in the General Education and Inclusion Science Classroom Gena M. Rosenzweig, Maria Carrodegaus, and Luretha Lucky Florida International University, USA Abstract: This study sought to apply the concepts of inquiry-based learning by increasing the number of laboratory experiments conducted in two science classes, and to identify the challenges of this instruction for students with special needs. Results showed that the grades achieved through lab write-ups greatly improved grades overall. The National Science Education Standards (1996) describes inquiry-based instruction as a way to involve students in a form of active learning that emphasizes questioning, data analysis, and critical thinking. This teaching practice encourages students to learn inductively and has a firmly established place in pedagogical tradition (Colburn, 2004). For example, theorists Johann Heinrich Pestalozzi and Herbert Spencer have championed student learning through concrete experiences and observations rather than rote memorization. Students at all grade levels, in every domain of science education, should be afforded the opportunity to use scientific inquiry to develop the ability to think and act in ways that are associated with inquiry (Bell, Smetana, & Binns, 2005). A convenient way to accomplish this goal in middle school classrooms is to enhance the inquiry aspects of activities that are already incorporated into the curriculum (Godbey, Barnett, & Webster, 2005). Teachers are challenged to effectively use inquiry-based learning strategies to motivate and teach students, particularly those in a special education curriculum. When properly introduced, inquiry-based activities increase student interest and motivation. The student becomes a fellow investigator, with motivation shifting from an extrinsic desire for a higher grade to an intrinsic one for satiating a curiosity of nature (Baker, Lang, & Lawson, 2002). -

A Handbook for Michigan Courts on Accessibility and Accommodation for Individuals with Disabilities
State Court Administrative Office Trial Court Services A Handbook for Michigan Courts on Accessibility and Accommodation for Individuals with Disabilities March 2018 i Table of Contents Acknowledgements ............................................................................................................. 1 Overview ............................................................................................................................. 1 Introduction ......................................................................................................................... 3 PART I An Overview of the Americans with Disabilities Act of 1990 (ADA) and the ADA Amendments Act of 2008. ............................................................................ 6 The Americans with Disabilities Act (ADA) ................................................................. 6 The ADA Amendments Act of 2008 (ADAAA) ............................................................ 6 Equal Opportunity ........................................................................................................... 6 Defining Disability Pursuant to the ADA ....................................................................... 7 Qualified Individual with a Disability ............................................................................ 8 Activities Covered by the ADA ...................................................................................... 9 Integrated Settings ......................................................................................................... -

Disability Policy Recommendations for the Biden Administration December 2020
Disability Policy Recommendations for the Biden Administration December 2020 Message from the Board The Consortium for Citizens with Disabilities (CCD) is pleased to CCD Board of Directors present our policy recommendations to the Biden Administration outlining the needs of people with disabilities and their families. Heather Ansley, CCD Chair Paralyzed Veterans of America CCD is the largest coalition of national organizations working [email protected] together to advocate for federal public policy that ensures the self-determination, independence, empowerment, integration, Laura Weidner, CCD Vice- and inclusion of children and adults with disabilities in all aspects Chair of society. Epilepsy Foundation [email protected] The COVID-19 pandemic has exacerbated the problems effecting people with disabilities, particularly those who are people of color. Sarah Meek, CCD Treasurer The pandemic has affected the policy areas under the purview of American Network of Community nearly every CCD task force. From decreased access to public Options and Resources transportation, to limited services for school-aged children with (ANCOR) disabilities, to concerns about health care rationing, and the [email protected] spread of the virus among individuals living in institutions, no area has gone unaffected. Carol Tyson, Secretary Disability Rights Education & Our recommendations seek to address the critical issues people Defense Fund (DREDF) with disabilities and their families are facing as our nation [email protected] continues to grapple with the effects of the pandemic. We look forward to working with your Administration to implement the Kim Musheno, Immediate Past solutions outlined in this document that we believe will ensure CCD Chair people with disabilities are able to live fuller lives in their Autism Society of America communities. -

NIC Seniors Housing Boot Camp: an Interactive Workshop on the Art Of
NIC Seniors Housing Boot Camp: An Interactive Workshop on the Art of Assessing a Deal Workshop Agenda: Presentations • Industry Overview • Introduction of Case Study • Market Assessment • Sales & Marketing • Care & Staffing • Investment Assessment & Operations Strategy • Financing Terms & Debt Placement • Review of Considerations & Valuation Table Discussions Value Review & Full Group Discussion Bre Grubbs SVP, New Business Development What is the Primary Reason for Your Attendance Today? a.Learn more about a particular portion of the acquisition process b.Learn more about how to value an opportunity c. Network and meet other new entrants to the industry d.I heard the snacks are pretty awesome Lana Peck Senior Principal • Real Estate Cycles • General Market Fundamentals Seniors Housing • Occupancy Today: • Inventory growth • Development • Asking Rent Growth • Transactions • Valuations Real Estate Cycles and Where Are We Today? Boom Hyper Supply Market Phase Declining Expansion Phase Occupancy Rising Occupancy Falling Values Rising Rents RAPID Rising Values Construction NEW Construction OVERBUILDING BUILD AND SELL Saturated Market Recovery Phase Recession Phase Rising Occupancy Falling Occupancy Rising Rents Falling Values Rising Values NO new construction NO new construction BUY Trough 7 Assisted Living Occupancy Far Lower Than Independent Living Occupancy Primary Markets | 1Q06-2Q17 IL AL Srs Hsg 93% 92% 91% 90% 89% 88% 87% 86% 85% 84% 83% 2Q2006 2Q2007 2Q2008 2Q2009 2Q2010 2Q2011 2Q2012 2Q2013 2Q2014 2Q2015 2Q2016 2Q2017 Source: NIC