Distinctive Supramolecular Features of -Cyclodextrin Inclusion Complexes with Antidepressants Protriptyline and Maprotiline
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
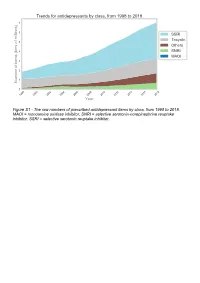
The Raw Numbers of Prescribed Antidepressant Items by Class, from 1998 to 2018
Figure S1 - The raw numbers of prescribed antidepressant items by class, from 1998 to 2018. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Figure S2 - The raw numbers of prescribed antidepressant items for the ten most commonly prescribed antidepressants (in 2018), from 1998 to 2018. Figure S3 - Practice level percentile charts for the proportions of the ten most commonly prescribed antidepressants (in 2018), prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S4 - Practice level percentile charts for the proportions of specific MAOIs prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S5 - CCG level heat map for the percentage proportions of MAOIs prescribed between December 2018 and November 2019. MAOI = monoamine oxidase inhibitor. Figure S6 - CCG level heat maps for the percentage proportions of paroxetine, dosulepin, and trimipramine prescribed between December 2018 and November 2019. Table S1 - Antidepressant drugs, by category. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Class Drug MAOI Isocarboxazid, moclobemide, phenelzine, tranylcypromine SNRI Duloxetine, venlafaxine SSRI Citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, -

Current P SYCHIATRY
Current p SYCHIATRY N ew Investigators Tips to manage and prevent discontinuation syndromes Informed tapering can protect patients when you stop a medication Sriram Ramaswamy, MD Shruti Malik, MBBS, MHSA Vijay Dewan, MD Instructor, department of psychiatry Foreign medical graduate Assistant professor Creighton University Department of psychiatry Omaha, NE University of Nebraska Medical Center Omaha, NE bruptly stopping common psychotropics New insights on psychotropic A —particularly antidepressants, benzodi- drug safety and side effects azepines, or atypical antipsychotics—can trigger a discontinuation syndrome, with: This paper was among those entered in the 2005 • rebound or relapse of original symptoms Promising New Investigators competition sponsored • uncomfortable new physical and psycho- by the Neuroleptic Malignant Syndrome Information Service (NMSIS). The theme of this year’s competition logical symptoms was “New insights on psychotropic drug safety and • physiologic withdrawal at times. side effects.” To increase health professionals’ awareness of URRENT SYCHIATRY 1 C P is honored to publish this peer- the risk of these adverse effects, this article reviewed, evidence-based article on a clinically describes discontinuation syndromes associated important topic for practicing psychiatrists. with various psychotropics and offers strategies to NMSIS is dedicated to reducing morbidity and anticipate, recognize, and manage them. mortality of NMS by improving medical and psychiatric care of patients with heat-related disorders; providing -

A Drug Repositioning Approach Identifies Tricyclic Antidepressants As Inhibitors of Small Cell Lung Cancer and Other Neuroendocrine Tumors
Published OnlineFirst September 26, 2013; DOI: 10.1158/2159-8290.CD-13-0183 RESEARCH ARTICLE A Drug Repositioning Approach Identifi es Tricyclic Antidepressants as Inhibitors of Small Cell Lung Cancer and Other Neuroendocrine Tumors Nadine S. Jahchan 1 , 2 , Joel T. Dudley 1 , Pawel K. Mazur 1 , 2 , Natasha Flores 1 , 2 , Dian Yang 1 , 2 , Alec Palmerton 1 , 2 , Anne-Flore Zmoos 1 , 2 , Dedeepya Vaka 1 , 2 , Kim Q.T. Tran 1 , 2 , Margaret Zhou 1 , 2 , Karolina Krasinska 3 , Jonathan W. Riess 4 , Joel W. Neal 5 , Purvesh Khatri 1 , 2 , Kwon S. Park 1 , 2 , Atul J. Butte 1 , 2 , and Julien Sage 1 , 2 Downloaded from cancerdiscovery.aacrjournals.org on September 29, 2021. © 2013 American Association for Cancer Research. Published OnlineFirst September 26, 2013; DOI: 10.1158/2159-8290.CD-13-0183 ABSTRACT Small cell lung cancer (SCLC) is an aggressive neuroendocrine subtype of lung cancer with high mortality. We used a systematic drug repositioning bioinformat- ics approach querying a large compendium of gene expression profi les to identify candidate U.S. Food and Drug Administration (FDA)–approved drugs to treat SCLC. We found that tricyclic antidepressants and related molecules potently induce apoptosis in both chemonaïve and chemoresistant SCLC cells in culture, in mouse and human SCLC tumors transplanted into immunocompromised mice, and in endog- enous tumors from a mouse model for human SCLC. The candidate drugs activate stress pathways and induce cell death in SCLC cells, at least in part by disrupting autocrine survival signals involving neurotransmitters and their G protein–coupled receptors. The candidate drugs inhibit the growth of other neuroendocrine tumors, including pancreatic neuroendocrine tumors and Merkel cell carcinoma. -

Hyponatremia and Psychotropic Drugs
DOI: 10.5772/intechopen.79029 ProvisionalChapter chapter 2 Hyponatremia and Psychotropic Drugs MireiaMireia Martínez Cortés Martínez Cortés and Pedro Gurillo MuñozPedro Gurillo Muñoz Additional information is available at the end of the chapter http://dx.doi.org/10.5772/intechopen.79029 Abstract Given the widespread use of psychotropic drugs in the population, it’s important to consider hyponatremia as an avoidable and reversible adverse effect and include the detection of high-risk subjects to establish safer medications, as well as early detection measures in routine clinical practice. Although hyponatremia has been especially associ- ated with serotonergic antidepressants (SSRIs), there is also an elevated risk with tricy- clics, duals and heterocyclic antidepressants, due to the different mechanisms of action at the renal tubular level and the release of ADH. Hyponatremia secondary to tricyclics with slow CYP2D6 metabolizers have higher plasma concentrations of antidepressants metabolized by CYP2D6. Hyponatremia secondary to SSRIs appears in the first week of treatment, it is “not dose-dependent” and normalization of natremia occurs between 2 and 20 days after stopping the medication. Bupropion, trazodone, mianserin, reboxetine and agomelatine are a safe alternative. Also antiepileptics have been related to hypona- tremia. Both typical and atypical antipsychotics have been exposed to an increased risk of hyponatremia, even after adjusted factors such as age, sex and comorbidity. Other factors that favor the onset of hyponatremia act synergistically with psychotropic drugs, such as: advanced age, female sex, concomitant diuretic intake, low body weight and low sodium levels; NSAID, ACEIs, and warm. Keywords: hyponatremia, antipsychotic, antidepressant, antiepileptic, psychotropic drugs 1. Introduction Hyponatremia is the most frequent hydroelectrolytic disorder in clinical practice, both in hospital and outpatient settings [1]. -

Fatal Toxicity of Antidepressant Drugs in Overdose
BRITISH MEDICAL JOURNAL VOLUME 295 24 OCTOBER 1987 1021 Br Med J (Clin Res Ed): first published as 10.1136/bmj.295.6605.1021 on 24 October 1987. Downloaded from PAPERS AND SHORT REPORTS Fatal toxicity of antidepressant drugs in overdose SIMON CASSIDY, JOHN HENRY Abstract dangerous in overdose, thus meriting investigation of their toxic properties and closer consideration of the circumstances in which A fatal toxicity index (deaths per million National Health Service they are prescribed. Recommendations may thus be made that prescriptions) was calculated for antidepressant drugs on sale might reduce the number offatalities. during the years 1975-84 in England, Wales, and Scotland. The We used national mortality statistics and prescription data tricyclic drugs introduced before 1970 had a higher index than the to compile fatal toxicity indices for the currently available anti- mean for all the drugs studied (p<0-001). In this group the depressant drugs to assess the comparative safety of the different toxicity ofamitriptyline, dibenzepin, desipramine, and dothiepin antidepressant drugs from an epidemiological standpoint. Owing to was significantly higher, while that ofclomipramine, imipramine, the nature of the disease these drugs are particularly likely to be iprindole, protriptyline, and trimipramine was lower. The mono- taken in overdose and often cause death. amine oxidase inhibitors had intermediate toxicity, and the antidepressants introduced since 1973, considered as a group, had significantly lower toxicity than the mean (p<0-001). Ofthese newer drugs, maprotiline had a fatal toxicity index similar to that Sources ofinformation and methods of the older tricyclic antidepressants, while the other newly The statistical sources used list drugs under their generic and proprietary http://www.bmj.com/ introduced drugs had lower toxicity indices, with those for names. -

Association of Selective Serotonin Reuptake Inhibitors with the Risk for Spontaneous Intracranial Hemorrhage
Supplementary Online Content Renoux C, Vahey S, Dell’Aniello S, Boivin J-F. Association of selective serotonin reuptake inhibitors with the risk for spontaneous intracranial hemorrhage. JAMA Neurol. Published online December 5, 2016. doi:10.1001/jamaneurol.2016.4529 eMethods 1. List of Antidepressants for Cohort Entry eMethods 2. List of Antidepressants According to the Degree of Serotonin Reuptake Inhibition eMethods 3. Potential Confounding Variables Included in Multivariate Models eMethods 4. Sensitivity Analyses eFigure. Flowchart of Incident Antidepressant (AD) Cohort Definition and Case- Control Selection eTable 1. Crude and Adjusted Rate Ratios of Intracerebral Hemorrhage Associated With Current Use of SSRIs Relative to TCAs eTable 2. Crude and Adjusted Rate Ratios of Subarachnoid Hemorrhage Associated With Current Use of SSRIs Relative to TCAs eTable 3. Crude and Adjusted Rate Ratios of Intracranial Extracerebral Hemorrhage Associated With Current Use of SSRIs Relative to TCAs. eTable 4. Crude and Adjusted Rate Ratios of Intracerebral Hemorrhage Associated With Current Use of Antidepressants With Strong Degree of Inhibition of Serotonin Reuptake Relative to Weak eTable 5. Crude and Adjusted Rate Ratios of Subarachnoid Hemorrhage Associated With Current Use of Antidepressants With Strong Degree of Inhibition of Serotonin Reuptake Relative to Weak eTable 6. Crude and Adjusted Rate Ratios of Intracranial Extracerebral Hemorrhage Associated With Current Use of Antidepressants With Strong Degree of Inhibition of Serotonin Reuptake Relative to Weak This supplementary material has been provided by the authors to give readers additional information about their work. © 2016 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 10/02/2021 eMethods 1. -

General Pharmacology
GENERAL PHARMACOLOGY Winners of “Nobel” prize for their contribution to pharmacology Year Name Contribution 1923 Frederick Banting Discovery of insulin John McLeod 1939 Gerhard Domagk Discovery of antibacterial effects of prontosil 1945 Sir Alexander Fleming Discovery of penicillin & its purification Ernst Boris Chain Sir Howard Walter Florey 1952 Selman Abraham Waksman Discovery of streptomycin 1982 Sir John R.Vane Discovery of prostaglandins 1999 Alfred G.Gilman Discovery of G proteins & their role in signal transduction in cells Martin Rodbell 1999 Arvid Carlson Discovery that dopamine is neurotransmitter in the brain whose depletion leads to symptoms of Parkinson’s disease Drug nomenclature: i. Chemical name ii. Non-proprietary name iii. Proprietary (Brand) name Source of drugs: Natural – plant /animal derivatives Synthetic/semisynthetic Plant Part Drug obtained Pilocarpus microphyllus Leaflets Pilocarpine Atropa belladonna Atropine Datura stramonium Physostigma venenosum dried, ripe seed Physostigmine Ephedra vulgaris Ephedrine Digitalis lanata Digoxin Strychnos toxifera Curare group of drugs Chondrodendron tomentosum Cannabis indica (Marijuana) Various parts are used ∆9Tetrahydrocannabinol (THC) Bhang - the dried leaves Ganja - the dried female inflorescence Charas- is the dried resinous extract from the flowering tops & leaves Papaver somniferum, P album Poppy seed pod/ Capsule Natural opiates such as morphine, codeine, thebaine Cinchona bark Quinine Vinca rosea periwinkle plant Vinca alkaloids Podophyllum peltatum the mayapple -

The Organic Chemistry of Drug Synthesis
The Organic Chemistry of Drug Synthesis VOLUME 2 DANIEL LEDNICER Mead Johnson and Company Evansville, Indiana LESTER A. MITSCHER The University of Kansas School of Pharmacy Department of Medicinal Chemistry Lawrence, Kansas A WILEY-INTERSCIENCE PUBLICATION JOHN WILEY AND SONS, New York • Chichester • Brisbane • Toronto Copyright © 1980 by John Wiley & Sons, Inc. All rights reserved. Published simultaneously in Canada. Reproduction or translation of any part of this work beyond that permitted by Sections 107 or 108 of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful. Requests for permission or further information should be addressed to the Permissions Department, John Wiley & Sons, Inc. Library of Congress Cataloging in Publication Data: Lednicer, Daniel, 1929- The organic chemistry of drug synthesis. "A Wiley-lnterscience publication." 1. Chemistry, Medical and pharmaceutical. 2. Drugs. 3. Chemistry, Organic. I. Mitscher, Lester A., joint author. II. Title. RS421 .L423 615M 91 76-28387 ISBN 0-471-04392-3 Printed in the United States of America 10 987654321 It is our pleasure again to dedicate a book to our helpmeets: Beryle and Betty. "Has it ever occurred to you that medicinal chemists are just like compulsive gamblers: the next compound will be the real winner." R. L. Clark at the 16th National Medicinal Chemistry Symposium, June, 1978. vii Preface The reception accorded "Organic Chemistry of Drug Synthesis11 seems to us to indicate widespread interest in the organic chemistry involved in the search for new pharmaceutical agents. We are only too aware of the fact that the book deals with a limited segment of the field; the earlier volume cannot be considered either comprehensive or completely up to date. -

DBL™ Promethazine Hydrochloride Injection BP
DBL™ Promethazine Hydrochloride Injection BP 1. NAME OF THE MEDICINE Promethazine hydrochloride 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each mL of the solution contains 25.0 mg promethazine hydrochloride, 0.10 mg disodium edetate, 1.30 microlitre glacial acetic acid, 27.2 mg sodium acetate and 1.32 mg sodium metabisulfite in water for injections. Excipient(s) with known effect Sodium metabisulfite For the full list of excipients, see section 6.1 List of Excipients. 3. PHARMACEUTICAL FORM Solution for injection. DBL™ Promethazine Hydrochloride Injection BP is a clear, colourless solution of pH 5.0 to 6.0. 4. CLINICAL PARTICULARS 4.1 Therapeutic Indications DBL™ Promethazine Hydrochloride Injection BP is indicated for the following conditions: Treatment of allergic reactions such as: uncomplicated allergic conditions of the immediate type, e.g., pruritus, urticaria and angioedema, when oral therapy is impossible or contraindicated. Treatment and prevention of vomiting including: motion sickness; drug induced nausea; prevention and control of nausea and vomiting associated with certain types of anaesthesia and surgery, such as procedures with a high incidence of post-operative vomiting (e.g., gynaecological surgery, strabismus or middle ear surgery, and electroconvulsive therapy); in patients with a past history of motion sickness or post- operative vomiting; and in patients in whom avoidance of vomiting is crucial (e.g., neurosurgery and eye surgery). Page 1 of 10 Promethazine has sedative effects and it is also used in: pre-operative, post-operative and obstetric (during labour) sedation. 4.2 Dose and Method of Administration Dosage Allergic conditions Adults: 25 mg to 50 mg by deep intramuscular injection or slow intravenous injection; may be repeated within two hours if necessary. -

Neuroleptic Malignant Syndrome, Electroconvulsive Therapy and Other Treatments
NEUROLEPTIC MALIGNANT SYNDROME, ELECTROCONVULSIVE THERAPY AND OTHER TREATMENTS JASSIN M. JOURIA, MD Dr. Jassin M. Jouria is a medical doctor, professor of academic medicine, and medical author. He graduated from Ross University School of Medicine and has completed his clinical clerkship training in various teaching hospitals throughout New York, including King’s County Hospital Center and Brookdale Medical Center, among others. Dr. Jouria has passed all USMLE medical board exams, and has served as a test prep tutor and instructor for Kaplan. He has developed several medical courses and curricula for a variety of educational institutions. Dr. Jouria has also served on multiple levels in the academic field including faculty member and Department Chair. Dr. Jouria continues to serve as a Subject Matter Expert for several continuing education organizations covering multiple basic medical sciences. He has also developed several continuing medical education courses covering various topics in clinical medicine. Recently, Dr. Jouria has been contracted by the University of Miami/Jackson Memorial Hospital’s Department of Surgery to develop an e-module training series for trauma patient management. Dr. Jouria is currently authoring an academic textbook on Human Anatomy & Physiology. Abstract Neuroleptic Malignant Syndrome is both rare and potentially fatal. Health clinicians need to recognize signs and symptoms and ask the right questions to make an accurate diagnosis and begin treatment. While this condition is not entirely understood, its symptoms are recognizable and typically easily resolved with little or no long-term impact to the patient when caught early. A treatment and management plan must be implemented. Pharmacotherapy has not been consistently effective in all case reports of neuroleptic malignant syndrome. -

New Zealand Data Sheet
1 PARNATE tranylcypromine film-coated tablets 10 mg New Zealand Data Sheet 1 PARNATE® (10 MG FILM-COATED TABLETS) PARNATE 10 mg film-coated tablets. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Parnate 10 mg film-coated tablets: each tablet contains tranylcypromine sulfate equivalent to 10 mg of tranylcypromine. Excipients with known effect: each tablet contains sucrose 6 mg. For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Parnate 10 mg film-coated tablets contain "geranium rose" coloured, biconvex, film-coated tablets. 4 CLINICAL PARTICULARS 4.1 Therapeutic indications Parnate is indicated for the treatment of symptoms of depressive illness especially where treatment with other types of anti-depressants has failed. It is not recommended for use in mild depressive states resulting from temporary situational difficulties. 4.2 Dose and method of administration Adults Begin with 20 mg a day given as 10 mg in the morning and 10 mg in the afternoon. If there is no satisfactory response after two weeks, add one more tablet at midday. Continue this dosage for at least a week. A dosage of 3 tablets a day should only be exceeded with caution. When a satisfactory response is established, dosage may be reduced to a maintenance level. Some patients will be maintained on 20 mg per day, some will need only 10 mg daily. If no improvement occurs, continued administration is unlikely to be beneficial. 1 2 PARNATE tranylcypromine film-coated tablets 10 mg When given together with a tranquilliser, the dosage of Parnate is not affected. When the medicine is given concurrently with electroconvulsive therapy, the recommended dosage is 10 mg twice a day during the series and 10 mg a day afterwards as maintenance therapy. -

United States Patent (19) 11 Patent Number: 6,060,642 Tecott Et Al
US006060642A United States Patent (19) 11 Patent Number: 6,060,642 Tecott et al. (45) Date of Patent: May 9, 2000 54 SEROTONIN 5-HT6 RECEPTOR KNOCKOUT Roth, Bryan L., et al., “Binding of Typical and Atypical MOUSE Antipsychotic Agents 5-Hydroxytryptamine-6 and 5-Hydroxytryptamine–7 Receptors'." The Journal Of Phar 75 Inventors: Laurence H. Tecott, San Francisco; macology and Experimental Therapeutics (1994) vol. 268, Thomas J. Brennan, San Carlos, both No. (3):1403–1410. of Calif. Ruat, Martial, et al., “A Novel Rat Serotonin (5-HT) 73 Assignee: The Regents of the University of Receptor: Molecular Cloning, Localization And Stimulation Of cAMP Accumulation,” Biochemical and Biophysical California, Oakland, Calif. Communications Research Communications (May 28, 1993) 21 Appl. No.: 09/132,388 vol. 193, No. (1):268–276. Saudou, Frédéric, et al., “5-Hydroxytryptamine Receptor 22 Filed: Aug. 11, 1998 Subtypes. In Vertebrates And Inverterbrates,” Neurochem Int. (1994) vol. 25, No. (6):503–532. Related U.S. Application Data Sleight, A.J., et al., “Effects of Altered 5-HT Expression. In 60 Provisional application No. 60/055.817, Aug. 15, 1997. The Rat: Functional Studies Using Antisense Oligonucle 51 Int. Cl." ............................. C12N 5700; C12N 15/12; otides,” Behavioural Brain Research (1996) vol. AO1K 67/027; G01N 33/15; CO7H 21/04 73:245-248. 52 U.S. Cl. ................................... 800/3; 800/18; 800/21; Tecott, Laurence H., et al., “Behavioral Genetics: Genes And 800/9; 435/172.1; 435/1723; 435/325; Aggressiveness,” Current Biology (1996) vol. 6, No. 435/455; 536/23.5 (3):238–240. 58 Field of Search ...................................