HEDIS Reference Guide for Primary Care
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Papers and Originals
BRITISH MEDICAL JOURNAL 19 FEBRUARY 1972 463 PAPERS AND ORIGINALS Drug Interaction: Inhibitory Effect of Neuroleptics on Metabolism of Tricyclic Antidepressants in Man LARS F. GRAM, KERSTIN FREDRICSON OVERO British Medical Journal, 1972, 1, 463-465 excretion (Anders, 1971). There are relatively few reports on interaction concerning neuroleptics or tricyclic antidepressants. Summary Chlorpromazine has both stimulatory and inhibitory effects on the metabolism of hexobarbitone in Total urinary excretion of radioactivity after oral or experimental animals of a test of (Riimke and Bout, 1960-1). Furthermore, chlorpromazine intravenous administration dose '4C-imi- accelerates the metabolism pramine was measured in were tested ofmeprobamate (Kato and Vassanelli, eight patients. They 1962). Tricyclic antidepressants have been shown to inhibit the before, during, and after treatment with neuroleptics. metabolism Excretion diminished while the were of tremorine and oxotremorine in rats in vitro and patients being in vivo (Hammer and Sjoqvist, 1967). It has also been found treated with perphenazine, haloperidol, or chlorproma- that zine, not treatment. desipramine hydrochloride inhibits the metabolism of though during flupenthixol amphetamine in isolated, perfused rat liver (Dingell and Bass, Total urinary excretion of radioactivity and plasma 1969). Pretreatment levels of metabolites and were measured with phenobarbitone decreased steady-state unchanged drug plasma levels of desipramine and nortriptyline in man (Sjoqvist in five patients after a test dose of 14C-nortriptyline. Each et was before and al. 1968). These findings were later confirmed in a twin study patient tested again during perphenazine (Alexanderson et al., 1969). Forrest et treatment. In all patients perphenazine treatment caused: al. (1970) found increased (1) decrease of total urinary excretion, (2) decreased urinary excretion of chlorpromazine metabolites when patients plasma level of metabolites, and (3) increased plasma were given additional treatment with phenobarbitone. -

Schizophrenia Care Guide
August 2015 CCHCS/DHCS Care Guide: Schizophrenia SUMMARY DECISION SUPPORT PATIENT EDUCATION/SELF MANAGEMENT GOALS ALERTS Minimize frequency and severity of psychotic episodes Suicidal ideation or gestures Encourage medication adherence Abnormal movements Manage medication side effects Delusions Monitor as clinically appropriate Neuroleptic Malignant Syndrome Danger to self or others DIAGNOSTIC CRITERIA/EVALUATION (PER DSM V) 1. Rule out delirium or other medical illnesses mimicking schizophrenia (see page 5), medications or drugs of abuse causing psychosis (see page 6), other mental illness causes of psychosis, e.g., Bipolar Mania or Depression, Major Depression, PTSD, borderline personality disorder (see page 4). Ideas in patients (even odd ideas) that we disagree with can be learned and are therefore not necessarily signs of schizophrenia. Schizophrenia is a world-wide phenomenon that can occur in cultures with widely differing ideas. 2. Diagnosis is made based on the following: (Criteria A and B must be met) A. Two of the following symptoms/signs must be present over much of at least one month (unless treated), with a significant impact on social or occupational functioning, over at least a 6-month period of time: Delusions, Hallucinations, Disorganized Speech, Negative symptoms (social withdrawal, poverty of thought, etc.), severely disorganized or catatonic behavior. B. At least one of the symptoms/signs should be Delusions, Hallucinations, or Disorganized Speech. TREATMENT OPTIONS MEDICATIONS Informed consent for psychotropic -

Opipramol and Trifluoperazine in the Treatment of Anxiety and Tension
A COMPARATIVE STUDY OF TWO ANTIHISTAMINES 395 In table VI are given the results of a question directed at discovering how effective the preferred treatment was compared to any other antihistamine used. Fifteen patients only were able to give this information but the results are interesting. Discussion TABLE VI The design of this study was very simple as COMPARISON WITH PREVIOUSLY USED ANTIHISTAMINE such studies must be if they are to be completed under the conditions of a busy general practice. In a condition such as hay fever where anti- Worse As good Better Total histamines are known to be etfective and where fairly rapid symptomatic relief is needed by the 2 4 9 15* patient, we did not feel justified in including a placebo. The results of the study seem fairly *In the majority of patients the previously used clear-cut that if patients are offered the choice antihistamine was the chemically related chlor- of a plain form of this antihistamine or a long- pheniramine maleate B.P. acting form more will choose the latter. Ofthose that do so, however, only about a third find a single tablet at night completely effective, the remainder have to take one further tablet during the day. Both forms of antihistamine are effective and where a retrospective comparison can be made this effectiveness is considered greater or equal to that of previously administered anti-histamines. Summary A simple comparative study under general-practice conditions of two formulations of pheniramine is described. Sixty-one per cent of patients preferred the long-acting form of this antihistamine. -
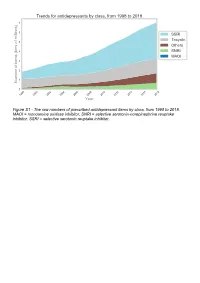
The Raw Numbers of Prescribed Antidepressant Items by Class, from 1998 to 2018
Figure S1 - The raw numbers of prescribed antidepressant items by class, from 1998 to 2018. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Figure S2 - The raw numbers of prescribed antidepressant items for the ten most commonly prescribed antidepressants (in 2018), from 1998 to 2018. Figure S3 - Practice level percentile charts for the proportions of the ten most commonly prescribed antidepressants (in 2018), prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S4 - Practice level percentile charts for the proportions of specific MAOIs prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S5 - CCG level heat map for the percentage proportions of MAOIs prescribed between December 2018 and November 2019. MAOI = monoamine oxidase inhibitor. Figure S6 - CCG level heat maps for the percentage proportions of paroxetine, dosulepin, and trimipramine prescribed between December 2018 and November 2019. Table S1 - Antidepressant drugs, by category. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Class Drug MAOI Isocarboxazid, moclobemide, phenelzine, tranylcypromine SNRI Duloxetine, venlafaxine SSRI Citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, -

NORPRAMIN® (Desipramine Hydrochloride Tablets USP)
NORPRAMIN® (desipramine hydrochloride tablets USP) Suicidality and Antidepressant Drugs Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of NORPRAMIN or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. NORPRAMIN is not approved for use in pediatric patients. (See WARNINGS: Clinical Worsening and Suicide Risk, PRECAUTIONS: Information for Patients, and PRECAUTIONS: Pediatric Use.) DESCRIPTION NORPRAMIN® (desipramine hydrochloride USP) is an antidepressant drug of the tricyclic type, and is chemically: 5H-Dibenz[bƒ]azepine-5-propanamine,10,11-dihydro-N-methyl-, monohydrochloride. 1 Reference ID: 3536021 Inactive Ingredients The following inactive ingredients are contained in all dosage strengths: acacia, calcium carbonate, corn starch, D&C Red No. 30 and D&C Yellow No. 10 (except 10 mg and 150 mg), FD&C Blue No. 1 (except 25 mg, 75 mg, and 100 mg), hydrogenated soy oil, iron oxide, light mineral oil, magnesium stearate, mannitol, polyethylene glycol 8000, pregelatinized corn starch, sodium benzoate (except 150 mg), sucrose, talc, titanium dioxide, and other ingredients. -

Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment for Schizophrenia in Adult Patients? Kyle J
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Student Dissertations, Theses and Papers Scholarship 2017 Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment For Schizophrenia In Adult Patients? Kyle J. Knowles Philadelphia College of Osteopathic Medicine Follow this and additional works at: https://digitalcommons.pcom.edu/pa_systematic_reviews Part of the Psychiatry Commons Recommended Citation Knowles, Kyle J., "Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment For Schizophrenia In Adult Patients?" (2017). PCOM Physician Assistant Studies Student Scholarship. 381. https://digitalcommons.pcom.edu/pa_systematic_reviews/381 This Selective Evidence-Based Medicine Review is brought to you for free and open access by the Student Dissertations, Theses and Papers at DigitalCommons@PCOM. It has been accepted for inclusion in PCOM Physician Assistant Studies Student Scholarship by an authorized administrator of DigitalCommons@PCOM. For more information, please contact [email protected]. Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment For Schizophrenia In Adult Patients? Kyle J. Knowles, PA-S A SELECTIVE EVIDENCE BASED MEDICINE REVIEW In Partial Fulfillment of the Requirements For The Degree of Master of Science In Health Sciences- Physician Assistant Department of Physician Assistant Studies Philadelphia College of Osteopathic Medicine Philadelphia, Pennsylvania December 16, 2016 ABSTRACT OBJECTIVE: The objective of this selective EBM review is to determine whether or not “Is Aristada (aripiprazole lauroxil) a safe and effective treatment for schizophrenia in adult patients?” STUDY DESIGN: Review of three randomized controlled studies. All three trials were conducted between 2014 and 2015. DATA SOURCES: One randomized, controlled trial and two randomized, controlled, double- blind trials found via Cochrane Library and PubMed. -

PERPHENAZINE and AMITRIPTYLINE HYDROCHLORIDE- Perphenazine and Amitriptyline Hydrochloride Tablet, Film Coated Mylan Pharmaceuticals Inc
PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE- perphenazine and amitriptyline hydrochloride tablet, film coated Mylan Pharmaceuticals Inc. ---------- WARNING Increased Mortality in Elderly Patients with Dementia-Related Psychosis Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug- treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Perphenazine and amitriptyline hydrochloride is not approved for the treatment of patients with dementia- related psychosis (see WARNINGS). Suicidality and Antidepressant Drugs Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of perphenazine and amitriptyline or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. -

Fatal Toxicity of Antidepressant Drugs in Overdose
BRITISH MEDICAL JOURNAL VOLUME 295 24 OCTOBER 1987 1021 Br Med J (Clin Res Ed): first published as 10.1136/bmj.295.6605.1021 on 24 October 1987. Downloaded from PAPERS AND SHORT REPORTS Fatal toxicity of antidepressant drugs in overdose SIMON CASSIDY, JOHN HENRY Abstract dangerous in overdose, thus meriting investigation of their toxic properties and closer consideration of the circumstances in which A fatal toxicity index (deaths per million National Health Service they are prescribed. Recommendations may thus be made that prescriptions) was calculated for antidepressant drugs on sale might reduce the number offatalities. during the years 1975-84 in England, Wales, and Scotland. The We used national mortality statistics and prescription data tricyclic drugs introduced before 1970 had a higher index than the to compile fatal toxicity indices for the currently available anti- mean for all the drugs studied (p<0-001). In this group the depressant drugs to assess the comparative safety of the different toxicity ofamitriptyline, dibenzepin, desipramine, and dothiepin antidepressant drugs from an epidemiological standpoint. Owing to was significantly higher, while that ofclomipramine, imipramine, the nature of the disease these drugs are particularly likely to be iprindole, protriptyline, and trimipramine was lower. The mono- taken in overdose and often cause death. amine oxidase inhibitors had intermediate toxicity, and the antidepressants introduced since 1973, considered as a group, had significantly lower toxicity than the mean (p<0-001). Ofthese newer drugs, maprotiline had a fatal toxicity index similar to that Sources ofinformation and methods of the older tricyclic antidepressants, while the other newly The statistical sources used list drugs under their generic and proprietary http://www.bmj.com/ introduced drugs had lower toxicity indices, with those for names. -

Heat Related Illness in Psychotropic Medication Users
Common psychotropic medications which Prevention of Heat can impair your response to heat Related Illness Trade Name Generic Name Abilify aripiprazole During periods of high temperature (85º Asendin amoxapine and above) and humidity, there are things Artane trihexyphenidyl everyone, particularly people at high risk, Aventil, Pamelor nortriptyline should do to lessen the chances of heat Clozaril clozapine illness. Cogentin benztropine Compazine prochlorperazine ¾ Try to stay cool. Desyrel trazodone • Stay in air conditioned areas if Elavil, Limbitrol, possible. If you do not have air Triavil amitriptyline conditioning at home, go to a Eskalith, Lithobid, shopping mall or public library. Lithonate lithium • Keep windows shut and draperies, Geodon ziprasidone shades, or blinds drawn during the Haldol haloperidol heat of the day. Loxitane loxapine • Open windows in the evening or Ludiomil maprotiline night hours when the air outside is Mellaril thioridazine Heat Related Illness cooler. Moban molindone • Move to cooler rooms during the Navane thiothixene in heat of the day. Norpramin desipramine Psychotropic ¾ Avoid overexertion and outdoor Phenergan promethazine activity, particularly during warmer Prolixin fluphenazine Medication Users periods of the day. Risperdal risperidone ¾ Apply sunscreen and lotion as needed. Serentil mesoridazine Seroquel quetiapine ¾ Drink plenty of fluids (avoid coffee, tea, and alcohol). Sinequan doxepin ¾ Dress in loose fitting, light colored Stelazine trifluoperazine clothing. Wear a hat, sunglasses, and Thorazine chlorpromazine other protective clothing. Tofranil imipramine ¾ Take a cool shower or bath. Trilafon perphenazine ¾ Lose weight if you are overweight. Wellbutrin buproprion ¾ Eat regular meals to ensure that you Zyprexa olanzapine Ohio Department of Mental Health have adequate salt and fluids. *Note: This is not an all inclusive list. -

Perphenazine Medical Facts from Drugs.Com Visited 10/07/2015
Perphenazine medical facts from Drugs.com http://www.drugs.com/mtm/perphenazine.html Visited 10/07/2015 Generic Name: perphenazine (per FEN a zeen) Brand Name: Trilafon What is perphenazine? Perphenazine is an anti-psychotic medicine in a group of drugs called phenothiazines (FEEN-oh-THYE-a-zeens). It works by changing the actions of chemicals in your brain. Perphenazine is used to treat psychotic disorders such as schizophrenia. It is also used to control severe nausea and vomiting. Perphenazine may also be used for purposes not listed in this medication guide. What is the most important information I should know about perphenazine? You should not use perphenazine if you have liver disease, brain damage, bone marrow depression, a blood cell disorder, or if you are also using large amounts of alcohol or medicines that make you sleepy. Call your doctor at once if you have twitching or uncontrollable movements of your eyes, lips, tongue, face, arms, or legs. These could be early signs of dangerous side effects. What should I discuss with my healthcare provider before taking perphenazine? You should not use this medicine if you are allergic to any phenothiazine (perphenazine, chlorpromazine, prochlorperazine, promethazine, thioridazine, Compazine, Phenergan, Mellaril, Thorazine, and others), or if you have: liver disease; brain damage; bone marrow depression; a blood cell disorder (such as low platelets or low red or white blood cell counts); or if you are also using large amounts of alcohol or medicines that make you sleepy. Perphenazine is not approved for use in psychotic conditions related to dementia. Perphenazine may increase the risk of death in older adults with dementia-related conditions. -

Association of Selective Serotonin Reuptake Inhibitors with the Risk for Spontaneous Intracranial Hemorrhage
Supplementary Online Content Renoux C, Vahey S, Dell’Aniello S, Boivin J-F. Association of selective serotonin reuptake inhibitors with the risk for spontaneous intracranial hemorrhage. JAMA Neurol. Published online December 5, 2016. doi:10.1001/jamaneurol.2016.4529 eMethods 1. List of Antidepressants for Cohort Entry eMethods 2. List of Antidepressants According to the Degree of Serotonin Reuptake Inhibition eMethods 3. Potential Confounding Variables Included in Multivariate Models eMethods 4. Sensitivity Analyses eFigure. Flowchart of Incident Antidepressant (AD) Cohort Definition and Case- Control Selection eTable 1. Crude and Adjusted Rate Ratios of Intracerebral Hemorrhage Associated With Current Use of SSRIs Relative to TCAs eTable 2. Crude and Adjusted Rate Ratios of Subarachnoid Hemorrhage Associated With Current Use of SSRIs Relative to TCAs eTable 3. Crude and Adjusted Rate Ratios of Intracranial Extracerebral Hemorrhage Associated With Current Use of SSRIs Relative to TCAs. eTable 4. Crude and Adjusted Rate Ratios of Intracerebral Hemorrhage Associated With Current Use of Antidepressants With Strong Degree of Inhibition of Serotonin Reuptake Relative to Weak eTable 5. Crude and Adjusted Rate Ratios of Subarachnoid Hemorrhage Associated With Current Use of Antidepressants With Strong Degree of Inhibition of Serotonin Reuptake Relative to Weak eTable 6. Crude and Adjusted Rate Ratios of Intracranial Extracerebral Hemorrhage Associated With Current Use of Antidepressants With Strong Degree of Inhibition of Serotonin Reuptake Relative to Weak This supplementary material has been provided by the authors to give readers additional information about their work. © 2016 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 10/02/2021 eMethods 1. -

Perphenazine-151548-PI.Pdf
PRESCRIBING INFORMATION PERPHENAZINE Perphenazine Tablets USP 2 mg , 4 mg, 8mg, and 16 mg Antipsychotic/Antiemetic AA Pharma Inc. DATE OF REVISION: April 12, 2012 1165 Creditsone Road, Unit #1 Vaughan, Ontario L4K 4N7 Control Number: 151548 PRESCRIBING INFORMATION PERPHENAZINE Perphenazine Tablets USP 2 mg, 4 mg, 8 mg and 16 mg THERAPEUTIC CLASSIFICATION Antipsychotic/Antiemetic ACTIONS AND CLINICAL PHARMACOLOGY Perphenazine is a piperazine phenothiazine derivative with antipsychotic, antiemetic and weak sedative activity. Perphenazine has actions similar to those of other phenothiazine derivatives but appears to be less sedating and to have a weak propensity for causing hypotension or potentiating the effects of CNS depressants and anesthetics. However, it produces a high incidence of extrapyramidal reactions. Perphenazine is well absorbed from the gastrointestinal tract. Onset of action following oral administration is 30 to 40 minutes. Duration of action is 3 to 4 hours. Perphenazine distributes to most body tissues with high concentrations being distributed into liver and spleen. Perphenazine enters the enterohepatic circulation and is excreted chiefly in the feces. INDICATIONS AND CLINICAL USE Perphenazine is indicated in the management of manifestations of psychotic disorders. It is also effective in controlling nausea and vomiting due to stimulation of the chemoreceptor trigger zone. 1 Perphenazine has not been shown effective for the management of behavioral complications in patients with mental retardation. CONTRAINDICATIONS Should not be administered in the presence of circulatory collapse, altered states of consciousness or comatose states, particularly when these are due to intoxication with central depressant drugs (alcohol, hypnotics, narcotics). It is contraindicated in severely depressed patients, in the presence of blood dyscrasias, liver disease, renal insufficiency, pheochromocytoma, or in patients with severe cardiovascular disorders or a history of hypersensitivity to phenothiazine derivatives.