2015-‐06-‐11 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Curative Pelvic Exenteration for Recurrent Cervical Carcinoma in the Era of Concurrent Chemotherapy and Radiation Therapy
Available online at www.sciencedirect.com ScienceDirect EJSO xx (2015) 1e11 www.ejso.com Review Curative pelvic exenteration for recurrent cervical carcinoma in the era of concurrent chemotherapy and radiation therapy. A systematic review H. Sardain a,b, V. Lavoue a,b,c,*, M. Redpath d, N. Bertheuil b,e, F. Foucher a,J.Lev^eque a,b,c a CHU de Rennes, Gynecology Department, Tertiary Surgery Center, Teaching Hospital of Rennes, Hopital^ Sud, 16, Bd de Bulgarie, 35000 Rennes, France b Universite de Rennes, Faculty of Medicine, 2 Henry Guilloux, 35000 Rennes, France c INSERM, ER440, Oncogenesis, Stress and Signaling (OSS), Rennes, France d McGill University, Department of Pathology, Jewish General Hospital, Cote^ Sainte Catherine, Montreal, QC, Canada e CHU de Rennes, Department of Plastic, Reconstructive and Aesthetic Surgery, Tertiary Surgery Center, Teaching Hospital of Rennes, Hopital^ Sud, 16, Bd de Bulgarie, 35000 Rennes, France Accepted 26 March 2015 Available online --- Abstract Objective: Pelvic exenteration requires complete resection of the tumor with negative margins to be considered a curative surgery. The pur- pose of this review is to assess the optimal preoperative evaluation and surgical approach in patients with recurrent cervical cancer to in- crease the chances of achieving a curative surgery with decreased morbidity and mortality in the era of concurrent chemoradiotherapy. Methods: Review of English publications pertaining to cervical cancer within the last 25 years were included using PubMed and Cochrane Library searches. Results: Modern imaging (MRI and PET-CT) does not accurately identify local extension of microscopic disease and is inadequate for pre- operative planning of extent of resection. -

Pelvic Exenteration for the Management of Pelvic Malignancies
Chapter 7 Pelvic Exenteration for the Management of Pelvic Malignancies Daniel Paramythiotis, Konstantinia Kofina and Antonios Michalopoulos Additional information is available at the end of the chapter http://dx.doi.org/10.5772/61083 Abstract Pelvic exenteration is a surgical procedure first described by Brunschwig in 1948 as a curative or palliative treatment for pelvic and perineal tumors. It is actually a radical operation, involving en bloc resection of pelvic organs, including reproductive structures, bladder, and rectosigmoid. In patients with recurrent cervical and vaginal malignancy, it is associated with a 5-year survival of more than 50%. In spite of advances in surgical management, consequences such as stomas, are still frequently unavoidable for radical tumor excision. Most candidates for this procedure have been diagnosed with recurrent cervical cancer that has previously been treated with surgery and radiation, or radiation alone. Complications of pelvic exenteration are more severe than those of standard resection of a colorectal carcinoma, so it is not commonly performed, including wound infection, wound dehiscence (also described as burst abdomen) the creation of fistulae (perineal-fecal, uretero-vaginal, between conduit and perineal wound), urinary tract infections, perineal hernias and intestinal obstruction. Patients need to be carefully selected and counseled about risks and long-term issues related to the surgery. A comprehensive evaluation is required in order to exclude unresectable or metastatic disease. Evolution of the technique through laparoscopy and minimally invasive surgery may result in a reduction of morbidity and mortality. Keywords: Pelvic exenteration, gynecologic cancer 1. Introduction Pelvic exenteration was first described by Brunschwig and his colleagues of New York’s Memorial Hospital in 1948 [1] and was initially performed as a palliative surgical intervention © 2015 The Author(s). -

FGM in Canada
Compiled by Patricia Huston MD, MPH Scientific Communications International, Inc for the Federal Interdepartmental Working Group on FGM. Copies of this report are available from: Women's Health Bureau Health Canada [email protected] The Canadian Women's Health Network 203-419 Graham Avenue Winnipeg, Manitoba R3C 0M3 fax: (204)989-2355 The opinions expressed in this report are not necessarily those of the Government of Canada or any of the other organizations represented. Dedication This report is dedicated to all the women in the world who have undergone FGM and to all the people who are helping them live with and reverse this procedure. This report is part of the ongoing commitment of Canadians and the Government of Canada to stop this practice in Canada and to improve the health and well-being of affected women and their communities. Executive Summary Female genital mutilation (FGM), or the ritual excision of part or all of the external female genitalia, is an ancient cultural practice that occurs around the world today, especially in Africa. With recent immigration to Canada of peoples from Somalia, Ethiopia and Eritrea, Sudan and Nigeria, women who have undergone this practice are now increasingly living in Canada. It is firmly believed by the people who practise it, that FGM improves feminine hygiene, that it will help eliminate disease and it is thought to be the only way to preserve family honour, a girl's virginity and her marriageability. FGM has a number of important adverse health effects including risks of infection and excessive bleeding (often performed when a girl is pre-pubertal). -

Treating Cervical Cancer If You've Been Diagnosed with Cervical Cancer, Your Cancer Care Team Will Talk with You About Treatment Options
cancer.org | 1.800.227.2345 Treating Cervical Cancer If you've been diagnosed with cervical cancer, your cancer care team will talk with you about treatment options. In choosing your treatment plan, you and your cancer care team will also take into account your age, your overall health, and your personal preferences. How is cervical cancer treated? Common types of treatments for cervical cancer include: ● Surgery for Cervical Cancer ● Radiation Therapy for Cervical Cancer ● Chemotherapy for Cervical Cancer ● Targeted Therapy for Cervical Cancer ● Immunotherapy for Cervical Cancer Common treatment approaches Depending on the type and stage of your cancer, you may need more than one type of treatment. For the earliest stages of cervical cancer, either surgery or radiation combined with chemo may be used. For later stages, radiation combined with chemo is usually the main treatment. Chemo (by itself) is often used to treat advanced cervical cancer. ● Treatment Options for Cervical Cancer, by Stage Who treats cervical cancer? Doctors on your cancer treatment team may include: 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 ● A gynecologist: a doctor who treats diseases of the female reproductive system ● A gynecologic oncologist: a doctor who specializes in cancers of the female reproductive system who can perform surgery and prescribe chemotherapy and other medicines ● A radiation oncologist: a doctor who uses radiation to treat cancer ● A medical oncologist: a doctor who uses chemotherapy and other medicines to treat cancer Many other specialists may be involved in your care as well, including nurse practitioners, nurses, psychologists, social workers, rehabilitation specialists, and other health professionals. -

Core Curriculum for Surgical Technology Sixth Edition
Core Curriculum for Surgical Technology Sixth Edition Core Curriculum 6.indd 1 11/17/10 11:51 PM TABLE OF CONTENTS I. Healthcare sciences A. Anatomy and physiology 7 B. Pharmacology and anesthesia 37 C. Medical terminology 49 D. Microbiology 63 E. Pathophysiology 71 II. Technological sciences A. Electricity 85 B. Information technology 86 C. Robotics 88 III. Patient care concepts A. Biopsychosocial needs of the patient 91 B. Death and dying 92 IV. Surgical technology A. Preoperative 1. Non-sterile a. Attire 97 b. Preoperative physical preparation of the patient 98 c. tneitaP noitacifitnedi 99 d. Transportation 100 e. Review of the chart 101 f. Surgical consent 102 g. refsnarT 104 h. Positioning 105 i. Urinary catheterization 106 j. Skin preparation 108 k. Equipment 110 l. Instrumentation 112 2. Sterile a. Asepsis and sterile technique 113 b. Hand hygiene and surgical scrub 115 c. Gowning and gloving 116 d. Surgical counts 117 e. Draping 118 B. Intraoperative: Sterile 1. Specimen care 119 2. Abdominal incisions 121 3. Hemostasis 122 4. Exposure 123 5. Catheters and drains 124 6. Wound closure 128 7. Surgical dressings 137 8. Wound healing 140 1 c. Light regulation d. Photoreceptors e. Macula lutea f. Fovea centralis g. Optic disc h. Brain pathways C. Ear 1. Anatomy a. External ear (1) Auricle (pinna) (2) Tragus b. Middle ear (1) Ossicles (a) Malleus (b) Incus (c) Stapes (2) Oval window (3) Round window (4) Mastoid sinus (5) Eustachian tube c. Internal ear (1) Labyrinth (2) Cochlea 2. Physiology of hearing a. Sound wave reception b. Bone conduction c. -
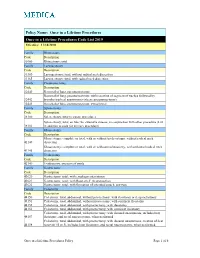
Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010
Policy Name: Once in a Lifetime Procedures Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010 Family Rhinectomy Code Description 30160 Rhinectomy; total Family Laryngectomy Code Description 31360 Laryngectomy; total, without radical neck dissection 31365 Laryngectomy; total, with radical neck dissection Family Pneumonectomy Code Description 32440 Removal of lung, pneumonectomy; Removal of lung, pneumonectomy; with resection of segment of trachea followed by 32442 broncho-tracheal anastomosis (sleeve pneumonectomy) 32445 Removal of lung, pneumonectomy; extrapleural Family Splenectomy Code Description 38100 Splenectomy; total (separate procedure) Splenectomy; total, en bloc for extensive disease, in conjunction with other procedure (List 38102 in addition to code for primary procedure) Family Glossectomy Code Description Glossectomy; complete or total, with or without tracheostomy, without radical neck 41140 dissection Glossectomy; complete or total, with or without tracheostomy, with unilateral radical neck 41145 dissection Family Uvulectomy Code Description 42140 Uvulectomy, excision of uvula Family Gastrectomy Code Description 43620 Gastrectomy, total; with esophagoenterostomy 43621 Gastrectomy, total; with Roux-en-Y reconstruction 43622 Gastrectomy, total; with formation of intestinal pouch, any type Family Colectomy Code Description 44150 Colectomy, total, abdominal, without proctectomy; with ileostomy or ileoproctostomy 44151 Colectomy, total, abdominal, without proctectomy; with continent ileostomy 44155 Colectomy, -

Pelvic Exenteration As Ultimate Ratio for Gynecologic Cancers: Single‑Center Analyses of 37 Cases
Archives of Gynecology and Obstetrics (2019) 300:161–168 https://doi.org/10.1007/s00404-019-05154-4 GYNECOLOGIC ONCOLOGY Pelvic exenteration as ultimate ratio for gynecologic cancers: single‑center analyses of 37 cases N. de Gregorio1 · A. de Gregorio1 · F. Ebner2 · T. W. P. Friedl1 · J. Huober1 · R. Hefty3 · M. Wittau4 · W. Janni1 · P. Widschwendter1 Received: 5 February 2019 / Accepted: 5 April 2019 / Published online: 22 April 2019 © Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract Background Pelvic exenterations are a last resort procedure for advanced gynecologic malignancies with elevated risks in terms of patients’ morbidity. Methods This single-center analysis reports surgical details, outcome and survival of all patients treated with exenteration for non-ovarian gynecologic malignancies at our university hospital during a 13-year time period. We collected data regarding patients and tumor characteristics, surgical procedures, peri- and postoperative management, transfusions, complications, and analyzed the impact on survival outcomes. Results We identifed 37 patients between 2005 and 2013 with primary or relapsed cervical cancer (59.5%), vulvar cancer (24.3%) or endometrial cancer (16.2%). Median age was 60 years and most patients (73%) had squamous cell carcinomas. Median progression-free survival was 26.2 months and median overall survival was 49.9 months. The 5-year survival rates were 34.4% for progression-free survival and 46.4% for overall survival. There were no signifcant diferences in progres- sion-free survival and overall survival with regard to disease entity. Patients with tumor at the resection margins (R1) had a nearly signifcantly worse progression-free survival (median: 28.5 vs. -

Seer Program Code Manual
THE SEER PROGRAM CODE MANUAL Revised Edition June 1992 CANCER STATISTICS BRANCH SURVEILLANCE PROGRAM DIVISION OF CANCER PREVENTION AND CONTROL NATIONAL CANCER INSTITUTE U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES PUBLIC HEALTH SERVICE NATIONAL INSTITUTES OF HEALTH Effective Date: Cases Diagnosed January 1, 1992 The SEER Program Code Manual Revised Edition June 1992 Editors Jack Cunningham Lynn Ries Benjamin Hankey Jennifer Seiffert Barbara Lyles Evelyn Shambaugh Constance Percy Valerie Van Holten Acknowledgements The editors wish to acknowledge the assistance of Terry Swenson, Maureen Troublefield, Diane Licitra, and Jerome Felix of Information Management Services, Inc., in the preparation of the SEER Program Code Manual. The editors also wish to acknowledge the assistance of Dr. John Berg in preparation of the section on multiple primary determination for lymphatic and hematopoietic diseases. TABLE OF CONTENTS PREFACE TO THE REVISED EDITION ....................................... vii COMPUTER RECORD FORMAT ............................................ 1 INTRODUCTION AND GENERAL INSTRUCTIONS .............................. 5 REFERENCES .......................................................... 37 SEER CODE SUMMARY .................................................. 39 I BASIC RECORD IDENTIFICATION ...................................... 57 1.01 SEER Participant ........................................... 58 1.02 Case Number .............................................. 59 1.03 Record Number ............................................ 60 -

Primary Pelvic Exenteration: Our Experience with 23 Patients from a Single Institution
EXPERIMENTAL AND THERAPEUTIC MEDICINE 22: 1060, 2021 Primary pelvic exenteration: Our experience with 23 patients from a single institution MIHAI GHEORGHE1, ALEXANDRA LAVINIA COZLEA1, SZILARD LEO KISS1, MIHAI STANCA1, MIHAI EMIL CĂPÎLNA1, NICOLAE BACALBAȘA2 and ANDREEA ANAMARIA MOLDOVAN3 1First Obstetrics and Gynecology Clinic, ‘George Emil Palade’ University of Medicine, Pharmacy, Science and Technology, 540136 Târgu Mureș; 2Department of Obstetrics and Gynecology, ‘Carol Davila’ University of Medicine and Pharmacy, 020022 Bucharest; 3Department of Infectious Diseases, Brașov County Emergency Hospital, 500326 Brașov, Romania Received May 24, 2021; Accepted June 23, 2021 DOI: 10.3892/etm.2021.10494 Abstract. This study was designed with an aim to share our Introduction experience of primary pelvic exenterations. The study included 23 patients with different types of pelvic cancer enrolled at a In patients with advanced primary or recurrent gyneco‑ single institution between November 2011 and July 2020. The logic (1), urologic or rectal cancers without metastatic disease, patient mean age was 55 years (range, 43‑72 years) and the extensive aggressive surgery such as pelvic exenteration may oncological indications included: Stage IVa cervical cancer be necessary for curative intent treatment (2). (11 cases, 48.9%), stage IVa endometrial cancer (1 case, 4.3%), Brunschwig was the first to describe pelvic exentera‑ stage IVa vaginal cancer (6 cases, 26%), stage IIIb bladder tion in the 1940s (3). Exenteration was initially considered cancer (3 cases, 13%), stage IIIc rectal cancer (1 case, 4.3%) as a palliative procedure for patients with extensive pelvic and undifferentiated pelvic sarcoma (1 case, 4.3%). Total, cancers, with an extremely high perioperative mortality rate anterior, and posterior pelvic exenterations were performed of 23%. -

GENDER DYSPHORIA TREATMENT Policy Number: 2016T0580A Effective Date: January 1, 2017
UnitedHealthcare® Commercial Medical Policy GENDER DYSPHORIA TREATMENT Policy Number: 2016T0580A Effective Date: January 1, 2017 Table of Contents Page Related Commercial Policy INSTRUCTIONS FOR USE .......................................... 1 Blepharoplasty, Blepharoptosis and Brow Ptosis BENEFIT CONSIDERATIONS ...................................... 1 Repair COVERAGE RATIONALE ............................................. 2 Botulinum Toxin A and B DEFINITIONS .......................................................... 3 Cosmetic and Reconstructive Procedures APPLICABLE CODES ................................................. 4 DESCRIPTION OF SERVICES ...................................... 8 Gonadotropin Releasing Hormone Analogs CLINICAL EVIDENCE ................................................. 8 Panniculectomy and Body Contouring Procedures U.S. FOOD AND DRUG ADMINISTRATION ................... 11 Rhinoplasty and Other Nasal Surgeries CENTERS FOR MEDICARE AND MEDICAID SERVICES ... 11 Speech Language Pathology Services REFERENCES .......................................................... 11 POLICY HISTORY/REVISION INFORMATION ................ 12 Community Plan Policy Gender Dysphoria Treatment Related Optum Guideline Gender Dysphoria INSTRUCTIONS FOR USE This Medical Policy provides assistance in interpreting UnitedHealthcare benefit plans. When deciding coverage, the member specific benefit plan document must be referenced. The terms of the member specific benefit plan document [e.g., Certificate of Coverage (COC), Schedule of -

April 2013, Volume 5, Issue 4
VOLUME 5 MONTHLY ISSUE MEDICAL STAFF 4 NEWSLETTER ProgressNotes April TORRANCE MEMORIAL MEDICAL CENTER 2013 this issue CPOE P.1 Doctor’s Day P.2 MEC Approvals P.3-4 Medical Staff Calendar P.5 New Providers P.6 Roster Updates P.6 What is it? CPOE Select Rewards is a tiered program geared to reward pro- CPOE viders as they expand their use of CPOE (Computerized Provider Order Entry). Computerized How are providers enrolled in the CPOE Select Rewards Provider Order Program? Entry Select Enrollment is automatic as you or your group begins CPOE. Rewards Program How does it work? Providers qualify for monthly raffles based on meeting or exceed- ing CPOE percentage goals for their assigned tier. When will winners be announced? Winners will be announced the second Monday of every month, beginning in April. CPOE Monthly Raffle includes several prizes including an i-Pad mini. Individualized program details will be sent out to providers. Please call CPOE hotline at x2763 (xCPOE) if you have any questions. Doctor’s Day Thank you to all the physicians who attended the Doctor’s Day Celebration at Torrance Memorial on Wednesday, March 27th. The event was a success with many physicians attending to enjoy a great meal, music and prizes. Congratulations to Dr. Lauren Nguyen and Dr. James Flores, the two lucky winners of the iPad mini and the thirty physicians who also won gift cards to Nordstroms, California Pizza Kitchen, HealthLinks, Starbucks and iTunes. Torrance Memorial appreciates our physicians’ dedication and commitment to the health of our community. Medical Executive Committee Approvals The following items were presented and actions were approved at the March 12, 2013 Medical Executive Committee meeting: A. -

Gender Dysphoria Treatment – Commercial Medical Policy
UnitedHealthcare® Commercial Medical Policy Gender Dysphoria Treatment Policy Number: 2021T0580J Effective Date: April 1, 2021 Instructions for Use Table of Contents Page Related Commercial Policies Coverage Rationale ....................................................................... 1 • Blepharoplasty, Blepharoptosis and Brow Ptosis Documentation Requirements ...................................................... 3 Repair Definitions ...................................................................................... 4 • Botulinum Toxins A and B Applicable Codes .......................................................................... 5 • Cosmetic and Reconstructive Procedures Description of Services ................................................................. 9 • Gonadotropin Releasing Hormone Analogs Benefit Considerations .................................................................. 9 Clinical Evidence ......................................................................... 10 • Habilitative Services and Outpatient Rehabilitation U.S. Food and Drug Administration ........................................... 15 Therapy References ................................................................................... 15 • Panniculectomy and Body Contouring Procedures Policy History/Revision Information ........................................... 16 • Rhinoplasty and Other Nasal Surgeries Instructions for Use ..................................................................... 17 Community Plan Policy • Gender Dysphoria