Neonatal Exposure to Sevoflurane Causes Apoptosis and Reduces Nnos Protein Expression in Rat Hippocampus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Veterinary Emergency & Anaesthesia Pfizer
AVA ECVA & AVEF Thank all their sponsors for this spring edition PARIS 2007 On VETERINARY EMERGENCY & ANAESTHESIA PFIZER MERIAL FORT DODGE BAYER BOEHRINGER MEDISUR COVETO OPTOMED HALLOWELL SCIL JANSSEN SOGEVAL KRUSSE TECHNIBELT MILA TEM The Organisatiors 7th AVEF European Meeting- 10th March 2007-ROISSY 2 AVA – ECVA Spring Meeting 2007 on Veterinary Emergency & Anesthesia 7 – 10 March 2007, Paris, France AVA PARIS 2007 — Wednesday March 7th RESIDENT DAY RUMINANT ANAESTHESIA Hyatt Regency Hotel, Roissy CDG, France K OTTO, D HOLOPHERNE, G TOUZOT 8.30 REGISTRATIONS 9.00-9.45 Specific anatomo-physiology to consider for ruminant peri-anaesthetic period K OTTO 10.00-10.30 COFFEE BREAK 10.30-11.15 Post-anaesthetic and pain management in ruminants K OTTO 11.30-12.15 Physical restraint and sedation of ruminants D HOLOPHERNE 12.30-1.30 LUNCH 1.30-2.15 Anaesthesia of Lamas & Alpagas G TOUZOT 2.30-3.15 Regional & local anaesthesia for ruminants D HOLOPHERNE 3.30-4.00 COFFEE BREAK 4.00-4.45 Pharmacology and protocols for ruminant anaesthesia G TOUZOT AVA-ECVA PARIS 2007, Veterinary Emergency & Anaesthesia, 7-10th March AVA-ECVA PARIS 2007, Veterinary Emergency & Anaesthesia, 7-10th March AVA – ECVA Spring Meeting 2007 on Veterinary Emergency & Anesthesia 7 – 10 March 2007, Paris, France Specific anatomo-physiology to consider for ruminants peri-anaesthetic period Klaus A. Otto Institut für Versuchstierkunde und Zentrales Tierlaboratorium, Medizinische Hochschule Hannover, D-30623 Hannover, Germany The suborder “ruminantia” includes members of the family “bovidae” such as cattle (bos taurus), sheep (ovis spp) and goats (capra spp). Members of the family “camelidae” (camelus spp, llama spp, vicugna spp) belong to the suborder “tylopodia” and therefore are not true ruminants. -

Pharmacology – Inhalant Anesthetics
Pharmacology- Inhalant Anesthetics Lyon Lee DVM PhD DACVA Introduction • Maintenance of general anesthesia is primarily carried out using inhalation anesthetics, although intravenous anesthetics may be used for short procedures. • Inhalation anesthetics provide quicker changes of anesthetic depth than injectable anesthetics, and reversal of central nervous depression is more readily achieved, explaining for its popularity in prolonged anesthesia (less risk of overdosing, less accumulation and quicker recovery) (see table 1) Table 1. Comparison of inhalant and injectable anesthetics Inhalant Technique Injectable Technique Expensive Equipment Cheap (needles, syringes) Patent Airway and high O2 Not necessarily Better control of anesthetic depth Once given, suffer the consequences Ease of elimination (ventilation) Only through metabolism & Excretion Pollution No • Commonly administered inhalant anesthetics include volatile liquids such as isoflurane, halothane, sevoflurane and desflurane, and inorganic gas, nitrous oxide (N2O). Except N2O, these volatile anesthetics are chemically ‘halogenated hydrocarbons’ and all are closely related. • Physical characteristics of volatile anesthetics govern their clinical effects and practicality associated with their use. Table 2. Physical characteristics of some volatile anesthetic agents. (MAC is for man) Name partition coefficient. boiling point MAC % blood /gas oil/gas (deg=C) Nitrous oxide 0.47 1.4 -89 105 Cyclopropane 0.55 11.5 -34 9.2 Halothane 2.4 220 50.2 0.75 Methoxyflurane 11.0 950 104.7 0.2 Enflurane 1.9 98 56.5 1.68 Isoflurane 1.4 97 48.5 1.15 Sevoflurane 0.6 53 58.5 2.5 Desflurane 0.42 18.7 25 5.72 Diethyl ether 12 65 34.6 1.92 Chloroform 8 400 61.2 0.77 Trichloroethylene 9 714 86.7 0.23 • The volatile anesthetics are administered as vapors after their evaporization in devices known as vaporizers. -

Federal Register/Vol. 71, No. 34/Tuesday, February 21, 2006
Federal Register / Vol. 71, No. 34 / Tuesday, February 21, 2006 / Notices 8859 DEPARTMENT OF HEALTH AND DEPARTMENT OF HEALTH AND of the Public Health Service Act to HUMAN SERVICES HUMAN SERVICES conduct directly or by grants or contracts, research, experiments, and Office of the National Coordinator; Office of the National Coordinator; demonstrations relating to occupational American Health Information American Health Information safety and health and to mine health. Community Chronic Care Workgroup Community Consumer Empowerment The BSC shall provide guidance to the Meeting Workgroup Meeting Director, NIOSH on research and prevention programs. Specifically, the ACTION: Announcement of meeting. ACTION: Announcement of meeting. board shall provide guidance on the institute’s research activities related to SUMMARY: SUMMARY: This notice announces the This notice announces the developing and evaluating hypotheses, third meeting of the American Health third meeting of the American Health Information Community Consumer systematically documenting findings Information Community Chronic Care and disseminating results. The board Workgroup in accordance with the Empowerment Workgroup in accordance with the Federal Advisory shall evaluate the degree to which the Federal Advisory Committee Act (Pub. activities of NIOSH: (1) Conform to L. 92–463, 5 U.S.C., App.) Committee Act (Pub. L. 92–463, 5 U.S.C., App.) appropriate scientific standards, (2) DATES: March 22, 2006 from 1 p.m. to address current, relevant needs, and (3) DATES: March 20, 2006 from 1 p.m. to 5 p.m. produce intended results. 5 p.m. ADDRESSES: Hubert H. Humphrey Matters to be Discussed: Agenda items ADDRESSES: Hubert H. Humphrey Building (200 Independence Ave., SW., include a report from the Director, Building (200 Independence Ave., SW., Washington, DC 20201), Conference NIOSH; progress report by BSC working Washington, DC 20201), Conference Room 705A. -

Anesthetics; Drugs of Abuse & Withdrawal
Anesthetics; Drugs of Abuse & Withdrawal Kurt Kleinschmidt, MD, FACEP, FACMT Professor of Emergency Medicine Section Chief and Program Director Medical Toxicology UT Southwestern Medical Center Much Thanks To… Sean M. Bryant, MD Associate Professor Cook County Hospital (Stroger) Department of Emergency Medicine Assistant Fellowship Director: Toxikon Consortium Associate Medical Director Illinois Poison Center Overview Anesthetics – Local – Inhalational – NM Blockers & Malignant Hyperthermia Drugs of Abuse (Pearls) Withdrawal History 1904-Procaine (short Duration of Action) 1925 (dibucaine) & 1928 (tetracaine) → potent, long acting 1943-lidocaine 1956-mepivacaine, 1959-prilocaine 1963-bupivacaine, 1971-etidocaine, 1996-ropivacaine Lipophili Intermediate Amine Substituents c Group Esters Structure 2 Distinct Groups 1) Amino Esters Amides 2) Amino Amides Local Anesthetics Toxic Reactions • Few & iatrogenic • Blood vessel administration or toxic dose AMIDES have largely replaced ESTERS • Increased stability • Relative absence of hypersensitivity reactions – ESTER hydrolysis = PABA (cross sensitivity) – AMIDES = Multidose preps → methylparabens • Chemically related to PABA with rare allergic reactions Local Anesthetics Mode of Action • Reversible & Predictable Binding • Within membrane-bound sodium channels of conducting tissue (cytoplasmic side of membrane) → Failure to form/propagate action potentials (Small-diam. fibersBLOCKADE carrying pain/temp sensation) Pain fibers - higher firing rate & longer AP → • ↑Sodium susceptible Channelto local -

Potentially Harmful Drugs for Mitochondrial Patients September 2016, Version 3
POTENTIALLY HARMFUL DRUGS FOR MITOCHONDRIAL PATIENTS SEPTEMBER 2016, VERSION 3 When the diagnosis of a mitochondrial disease is made, you (as a patient) may be confronted with medication/drugs to be used. Up to now there is no treatment for mitochondrial disorders. There are no therapies which can solve the primary problem: the lack of energy. However, it is possible to deal with specific complaints with so called symptomatic treatments. For example: a mitochondrial disorder can lead to epileptic seizures, which can be treated with anti-epileptics or in case of cardiomyopathy (when the heart muscle is affected) specific heart medication can be given. Additionally, you can be confronted with medication when you have to undergo surgery or medical investigation and need anaesthesia. It is of the utmost importance to realise that certain drugs may be potentially harmful for patients with mitochondrial disorders. The cause of the possible larger risk of unwanted negative effects of certain drugs with mitochondrial disorders in general lies in the fact that the drugs have a negative impact on the mitochondrial function. The (group of) drugs of which it is scientifically known that there is an (possible) increased risk on harmful effects with mitochondrial patients are listed in the table below. The kind of scientific evidence for negative effects on the mitochondrial function differs per (group of) drugs. We labelled the (group of) drugs based on the kind of scientific evidence, while we do not aim to restrict important drugs in a condition where treatment options are already so limited. In the majority it concerns experimental data, marked as yellow. -

Supplemental Anesthesia & Analgesia Information This Supplemental
Supplemental Anesthesia & Analgesia Information This supplemental information was prepared by members of the Veterinary Task Force to Advance Spay-Neuter (VTFASN) as a companion piece to the Association of Shelter Veterinarian’s Veterinary Medical Care Guidelines for Spay-Neuter Programs (Guidelines). Anesthesia and Analgesia Guidelines for High Quality, High Volume Spay/Neuter Initiatives Andrea L. Looney, DVM, DACVA, Leslie D. Appel, DVM, Mark W. Bohling, DVM, PhD, DACVS, Y. Karla Rigdon-Brestle, DVM, Philip A. Bushby, DVM, MS, DACVS, Nancy J. Ferguson, DVM, Brenda Griffin, DVM, MS, DACVIM, David J. Sweeney, DVM, Kathy A. Tyson, DVM, Adriana H. Voors, DVM, Sara C. White, DVM; Edited by Joan E. Dempsey, MFA INTRODUCTION As a recent ad campaign for inhalant anesthesia stated, “There’s a lot more to good anesthesia than simply life and death or waking from the event (1).” In other words, the fact that an animal makes it through surgery is no longer a good criterion by which to measure adequacy in our choices of anesthetic drugs, monitoring or stabilization. We understand now that what we have done or failed to do pre-, intra- or postoperatively has sometimes caused animals to succumb to perioperative disease within weeks or months of the surgery. Our historical presumptions of successful anesthesia, indicated by animals that appear stable under anesthesia or wake up quickly or well, are no longer adequate. Why is this so? The answer is relatively simple. Despite all our advancements within the fields of both human and veterinary anesthesiology, and regardless of whether we use simple mask inhalants or potent premeds such as Xylazine, anesthesia remains a profound cardiorespiratory depressant event which is capable of causing disease and death. -

Substitution of Isoflurane by Sevoflurane Toward the End of Long Surgeries Is Cost Effective
Alexandria Journal of Anaesthesia and Intensive Care 24 Substitution of Isoflurane by Sevoflurane Toward the End of Long Surgeries Is Cost Effective Moatz El-Tawil, Mohamed Yasser El Bahar Faculty of Medicine - Menofiya University ABSTRACT The lower solubility of sevoflurane allows a more rapid emergence from anesthesia than after anesthesia with more soluble but less expensive anesthetic isoflurane. Cost control in anesthesia is no longer an option; it is a necessity. We substitute sevoflurane for isoflurane toward the end of anesthesia for operations longer than 3 hours in an attempt to combine the cost effectiveness of isoflurane with rapid emergence from sevoflurane. Sixty patients undergoing long abdominoplastic and ENT surgeries were randomly equally divided into three groups: group I (isoflurane group), group II (crossover group) where isoflurane was substituted by sevoflurane during the last 30 minutes of the operation and group III (sevoflurane group). A fresh gas flow of 2 L/min as 60% N2O in O2 was used for maintenance of anesthesia. Consumption of volatile anesthetics was measured by weighing the vaporizers with a precision weighing machine and recovery variables were recorded. The times for spontaneous breathing, times to opening eyes, squeeze a finger on command, times for extubation, orientation, times to read Aldrete score > 9 and time to discharge from PACU, all these times were significantly longer in isoflurane group than the crossover and sevoflurane groups and no significant difference between crossover and isoflurane groups. Cost was significantly higher in sevoflurane group (1.242 EP per minute anesthesia). The costs among the other two groups did not differ significantly (0.319 EP/min for isoflurane group and 0.344 EP/min for crossover group). -

1 Comparison of the Effects of Buprenorphine and Methadone in 1
1 Comparison of the effects of buprenorphine and methadone in 2 combination with medetomidine followed by intramuscular alfaxalone for 3 anaesthesia of cats undergoing ovariohysterectomy. 4 5 Corresponding author: Kate L White, MA VetMB, DVA, Dipl ECVAA, MRCVS, 6 School of Veterinary Medicine and Science, University of Nottingham, 7 Loughborough, LE12 5RD, UK 8 e-mail: [email protected] 9 Tel: +44 (0)1159516096 10 11 12 Authors: 13 Mahdmina A, RSPCA Greater Manchester Animal Hospital, 411 Eccles New 14 Rd, Salford, M5 5NN 15 16 Evans, A, Rutland House Veterinary Hospital, 4 Abbotsfield Road, St. Helens, 17 WA9 4HU 18 19 Yates D, RSPCA Greater Manchester Animal Hospital, 411 Eccles New Rd, 20 Salford, M5 5NN 1 21 22 White KL, School of Veterinary Medicine and Science, University of Nottingham, 23 Loughborough, LE12 5RD, UK 24 25 Abstract 26 Objectives: The aim of this study was to compare quality of anaesthesia and 27 analgesia between methadone and buprenorphine in combination with 28 medetomidine after induction with intra-muscular (IM) alfaxalone in cats 29 undergoing ovariohysterectomy. 30 Methods: Fifty-one female cats (ASA I - II), median age 12 months (range 2 – 60), 31 weighing 2.5 ± 0.5 kg were recruited to the study. Cats were randomly allocated 32 to receive medetomidine (600 µg/m2) and buprenorphine (180 µg/m2) (group 33 MB) or medetomidine (500 µg/m2) and methadone (5 mg/m2) (group MM) IM. 34 Anaesthesia was induced 15 minutes later using alfaxalone (3 mg/kg) IM. 35 Anaesthesia was maintained with isoflurane in oxygen. All cats received 36 meloxicam pre-operatively. -

Comparison of Sevoflurane and Halothane Masaki Yurino MD Phl), Hitomi Kirnura MD
440 Vital capacity rapid inhalation induction technique: comparison of sevoflurane and halothane Masaki Yurino MD Phl), Hitomi Kirnura MD Induction of anaesthesia using the vital capacity rapid inhal- de cette dpreuve et chacun d'eux a refu un des deux agents: ation induction (VCRI1) technique with either sevoflurane or 17 ont refu le sdvoflurane et 15, l~alothane. Non pr~m~diquds, halothane was compared. The induction time, characteristics, ils ont inspir~ approximativement 2,6 fois l~quivalent de la and acceptability were assessed. Thirty-two volunteers were concentration alvdolaire minimum (CAM) de l'un ou de l'autre given one of the vapours: 17 received sevoflurane and 15 halo- des agents. II n'y avait de differences entre les parambtres cardio- thane. Subjects were unpremedicated and breathed approxi- vasculaires et respiratoires. La dur~e moyenne de l'induction mately 2.6 • minimum alveolar concentration (MAC) equiv- de l'anesth~sie avec l'halothane (153 + 46 sec, SD) a dtd plus alent of either agent. There were no differences in the patients' lente que celle produite par le s~voflurane (81 + 22 sec, SD, cardiovascular or respiratory variables. The mean time for in- P < 0,05), ce qui reflbte son coefficient de solubilit$ sang:gaz duction of anaesthesia with halothane (153 + 46 sec, SD) was plus dlev~. L~ncidence des complications telles que la toux et slower than with sevoflurane (81 + 22 sec, SD, P < 0.05), les mouvements a dt~ moindre avec s$voflurane qu'avec l~a- reflecting its higher blood:gas solubility. There were fewer in- lothane. -

Sevoflurane, Desflurane, and Xenon New Inhaled Anesthetics in Veterinary Medicine
Ciência Rural, Santa Maria, v.31, n.1, p.177-183, 2001 177 ISSN 0103-8478 SEVOFLURANE, DESFLURANE, AND XENON NEW INHALED ANESTHETICS IN VETERINARY MEDICINE SEVOFLURANO, DESFLURANO E XENÔNIO NOVOS ANESTÉSICOS INALATÓRIOS EM MEDICINA VETERINÁRIA Cláudio Correa Natalini 1 - REVIEW - SUMMARY relação ao uso clínico e a segurança do uso desses novos fármacos em animais. A literatura disponível sobre o uso desses Inhalation anesthesia is widely used in veterinary anestésicos em animais está revisada neste artigo. medicine. New inhalation anesthetics that present less untoward effects, are more potent and produce a safe and easily changeable Palavras-chave: anestesia inalatória, sevoflurano, desflurano, anesthetic plane are desirable over the older agents presently xenônio. available. In this review some of the physical and chemical aspects of inhalation anesthesia is revisited. Because the agents used in inhalation anesthesia are gases or vapors, the physics of vaporization, delivery and administration of these agents should INTRODUCTION be understood. The two new inhalation anesthetics sevoflurane and desflurane, and the new anesthetic gas xenon have been used Inhalation anesthetics are used widely for in human beings for some time. In veterinary medicine there is a the anesthetic management of animals. They are lack of investigation and reports that assure the safety and clinical aspects of using them in animals. The information unique among the anesthetic drugs because they are available on the use of these new agents in animals is revised in administered, and in large part removed from the this article. body, via the lungs. Their popularity arises in part because their pharmacokinetic characteristics favor Key words: inhalation anesthesia, sevoflurane, desflurane, and xenon. -

Cardiovascular Effects of Tramadol in Dogs Anesthetized with Sevoflurane
FULL PAPER Surgery Cardiovascular Effects of Tramadol in Dogs Anesthetized with Sevoflurane Takaharu ITAMI1), Naomichi TAMARU1), Kodai KAWASE1), Tomohito ISHIZUKA1), Jun TAMURA1), Kenjirou MIYOSHI1), Mohammed A. UMAR1), Hiroki INOUE2) and Kazuto YAMASHITA1)* 1)Department of Small Animal Clinical Sciences, School of Veterinary Medicine and 2)Department of Biosphere and Environmental Sciences, Faculty of Environment Systems, Rakuno Gakuen University, Ebetsu, Hokkaido 069–8501, Japan (Received 11 May 2011/Accepted 28 July 2011/Published online in J-STAGE 11 August 2011) ABSTRACT. Cardiovascular effects of tramadol were evaluated in dogs anesthetized with sevoflurane. Six beagle dogs were anesthetized twice at 7 days interval. The minimum alveolar concentration (MAC) of sevoflurane was earlier determined in each dog. The dogs were then anesthetized with sevoflurane at 1.3 times of predetermined individual MAC and cardiovascular parameters were evaluated before (baseline) and after an intravenous injection of tramadol (4 mg/kg). The administration of tramadol produced a transient and mild increase in arterial blood pressure (ABP) (P=0.004) with prolonged increase in systemic vascular resistance (SVR) (P<0.0001). Com- pared with baseline value, mean ABP increased significantly at 5 min (119% of baseline value, P=0.003), 10 min (113%, P=0.027), and 15 min (111%, P=0.022). SVR also increased significantly at 5 min (128%, P<0.0001), 10 min (121%, P=0.026), 30 min (114%, P=0.025), 45 min (113%, P=0.025) and 60 min (112%, P=0.048). Plasma concentrations of tramadol were weakly correlated with the percentage changes in mean ABP (r=0.642, P<0.0001) and SVR (r=0.646, P<0.0001). -
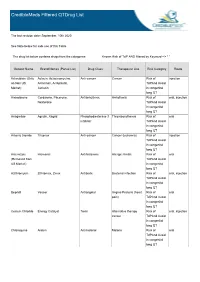
Crediblemeds Filtered Qtdrug List
CredibleMeds Filtered QTDrug List The last revision date: September, 10th 2020 See Note below for safe use of this Table The drug list below contains drugs from the categories: Known Risk of TdP AND filtered by Keyword --> " " Generic Name Brand Names (Partial List) Drug Class Therapeutic Use Risk Category Route Aclarubicin (Only Aclacin, Aclacinomycine, Anti-cancer Cancer Risk of injection on Non US Aclacinon, Aclaplastin, TdPAnd Avoid Market) Jaclacin in congenital long QT Amiodarone Cordarone, Pacerone, Antiarrhythmic Arrhythmia Risk of oral, injection Nexterone TdPAnd Avoid in congenital long QT Anagrelide Agrylin, Xagrid Phosphodiesterase 3 Thrombocythemia Risk of oral inhibitor TdPAnd Avoid in congenital long QT Arsenic trioxide Trisenox Anti-cancer Cancer (leukemia) Risk of injection TdPAnd Avoid in congenital long QT Astemizole Hismanal Antihistamine Allergic rhinitis Risk of oral (Removed from TdPAnd Avoid US Market) in congenital long QT Azithromycin Zithromax, Zmax Antibiotic Bacterial infection Risk of oral, injection TdPAnd Avoid in congenital long QT Bepridil Vascor Antianginal Angina Pectoris (heart Risk of oral pain) TdPAnd Avoid in congenital long QT Cesium Chloride Energy Catalyst Toxin Alternative therapy Risk of oral, injection cancer TdPAnd Avoid in congenital long QT Chloroquine Aralen Antimalarial Malaria Risk of oral TdPAnd Avoid in congenital long QT Generic Name Brand Names (Partial List) Drug Class Therapeutic Use Risk Category Route Chlorpromazine Thorazine, Largactil, Antipsychotic / Nausea, Schizophrenia,