Discrete Phenotypes Are Not Underpinned by Genome-Wide Genetic Differentiation in the Squat Lobster Munida Gregaria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Squat Lobsters of the Genus Munida (Crustacea: Decapoda: Anomura: Munididae) from the Ogasawara Islands, with Descriptions of Four New Species
国立科博専報,(47): 339–365,2011年4月15日 Mem. Natl. Mus. Nat. Sci., Tokyo, (47): 339–365, April 15, 2011 Squat Lobsters of the Genus Munida (Crustacea: Decapoda: Anomura: Munididae) from the Ogasawara Islands, with Descriptions of Four New Species Tomoyuki Komai Natural History Museum and Institute, Chiba, 955–2 Aoba-cho, Chuo-ku, Chiba-shi, Chiba 260–8682, Japan E-mail: [email protected] Abstract. The present study reports on the squat lobster genus Munida Leach, 1820 (Anomura: Munididae) collected in the Ogasawara Islands during the Project “Studies on the Origin of Bio- diversity in the Sagami Sea Fossa Magna Element and the Izu-Ogasawara (Bonin) Arc” in 2006–2010, carried out by the National Museum and Nature and Science. Six species were iden- tified, including four new species: M. disiunctus sp. nov., M. honshuensis Benedict, 1902, M. koyo sp. nov., M. longinquus sp. nov., M. munin sp. nov., and M pectinata Macpherson and Ma- chordom, 2005. The two previously described species are newly recorded from the area, of them M. pectinata is first recorded from waters outside New Caledonia. Affinities of the four new spe- cies are discussed. Key words: Crustacea, Munididae, Munida, new species, Ogasawara Islands Pacific (e.g., Baba, 1988; 1994; 2005; Baba et al., Introduction 2009; Macpherson, 1993; 1994; 1996a; 1996b; The galatheoid fauna of the oceanic Ogasawara 1997; 1999a; 1999b; 2000; 2004; 2006a; 2006b; Islands, located at about 1000 km south of Tokyo, 2009; Macpherson and de Saint Laurent, 1991; central Japan, is little known, although some pub- Macpherson and Baba, 1993; Macpherson and lications have been published (Stimpson, 1858; Machordom, 2005; Machordom and Macpherson, Balss, 1913; Melin, 1939; Miyake and Baba, 2004; Ahyong and Poore, 2004; Ahyong, 2007). -
A New Species of Squat Lobster of the Genus Hendersonida (Crustacea, Decapoda, Munididae) from Papua New Guinea
ZooKeys 935: 25–35 (2020) A peer-reviewed open-access journal doi: 10.3897/zookeys.935.51931 RESEARCH ARTICLE https://zookeys.pensoft.net Launched to accelerate biodiversity research A new species of squat lobster of the genus Hendersonida (Crustacea, Decapoda, Munididae) from Papua New Guinea Paula C. Rodríguez-Flores1,2, Enrique Macpherson1, Annie Machordom2 1 Centre d’Estudis Avançats de Blanes (CEAB-CSIC), C. acc. Cala Sant Francesc 14 17300 Blanes, Girona, Spain 2 Museo Nacional de Ciencias Naturales (MNCN-CSIC), José Gutiérrez Abascal, 2, 28006 Madrid, Spain Corresponding author: Paula C. Rodríguez-Flores ([email protected]) Academic editor: I.S. Wehrtmann | Received 10 March 2020 | Accepted 2 April 2020 | Published 21 May 2020 http://zoobank.org/E2D29655-B671-4A4C-BCDA-9A8D6063D71D Citation: Rodríguez-Flores PC, Macpherson E, Machordom A (2020) A new species of squat lobster of the genus Hendersonida (Crustacea, Decapoda, Munididae) from Papua New Guinea. ZooKeys 935: 25–35. https://doi. org/10.3897/zookeys.935.51931 Abstract Hendersonida parvirostris sp. nov. is described from Papua New Guinea. The new species can be distin- guished from the only other species of the genus, H. granulata (Henderson, 1885), by the fewer spines on the dorsal carapace surface, the shape of the rostrum and supraocular spines, the antennal peduncles, and the length of the walking legs. Pairwise genetic distances estimated using the 16S rRNA and COI DNA gene fragments indicated high levels of sequence divergence between the new species and H. granulata. Phylogenetic analyses, however, recovered both species as sister species, supporting monophyly of the genus. Keywords Anomura, mitochondrial genes, morphology, West Pacific Introduction Squat lobsters of the family Munididae Ahyong, Baba, Macpherson & Poore, 2010 are recognised by the trispinose or trilobate front, usually composed of a slender rostrum flanked by supraorbital spines (Ahyong et al. -

Deep-Water Squat Lobsters (Crustacea: Decapoda: Anomura) from India Collected by the FORV Sagar Sampada
Bull. Natl. Mus. Nat. Sci., Ser. A, 46(4), pp. 155–182, November 20, 2020 Deep-water Squat Lobsters (Crustacea: Decapoda: Anomura) from India Collected by the FORV Sagar Sampada Vinay P. Padate1, 2, Shivam Tiwari1, 3, Sherine Sonia Cubelio1,4 and Masatsune Takeda5 1Centre for Marine Living Resources and Ecology, Ministry of Earth Sciences, Government of India. Atal Bhavan, LNG Terminus Road, Puthuvype, Kochi 682508, India 2Corresponding author: [email protected]; https://orcid.org/0000-0002-2244-8338 [email protected]; https://orcid.org/0000-0001-6194-8960 [email protected]; http://orcid.org/0000-0002-2960-7055 5Department of Zoology, National Museum of Nature and Science, Tokyo. 4–1–1 Amakubo, Tsukuba, Ibaraki 305–0005, Japan. [email protected]; https://orcid/org/0000-0002-0028-1397 (Received 13 August 2020; accepted 23 September 2020) Abstract Deep-water squat lobsters collected during five cruises of the Fishery Oceanographic Research Vessel Sagar Sampada off the Andaman and Nicobar Archipelagos (299–812 m deep) and three cruises in the southeastern Arabian Sea (610–957 m deep) are identified. They are referred to each one species of the families Chirostylidae and Sternostylidae in the Superfamily Chirostyloidea, and five species of the family Munidopsidae and three species of the family Muni- didae in the Superfamily Galatheoidea. Of altogether 10 species of 5 genera dealt herein, the Uro- ptychus species of the Chirostylidae is described as new to science, and Agononida aff. indocerta Poore and Andreakis, 2012, of the Munididae, previously reported from Western Australia and Papua New Guinea, is newly recorded from Indian waters. -

Annotated Checklist of New Zealand Decapoda (Arthropoda: Crustacea)
Tuhinga 22: 171–272 Copyright © Museum of New Zealand Te Papa Tongarewa (2011) Annotated checklist of New Zealand Decapoda (Arthropoda: Crustacea) John C. Yaldwyn† and W. Richard Webber* † Research Associate, Museum of New Zealand Te Papa Tongarewa. Deceased October 2005 * Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, New Zealand ([email protected]) (Manuscript completed for publication by second author) ABSTRACT: A checklist of the Recent Decapoda (shrimps, prawns, lobsters, crayfish and crabs) of the New Zealand region is given. It includes 488 named species in 90 families, with 153 (31%) of the species considered endemic. References to New Zealand records and other significant references are given for all species previously recorded from New Zealand. The location of New Zealand material is given for a number of species first recorded in the New Zealand Inventory of Biodiversity but with no further data. Information on geographical distribution, habitat range and, in some cases, depth range and colour are given for each species. KEYWORDS: Decapoda, New Zealand, checklist, annotated checklist, shrimp, prawn, lobster, crab. Contents Introduction Methods Checklist of New Zealand Decapoda Suborder DENDROBRANCHIATA Bate, 1888 ..................................... 178 Superfamily PENAEOIDEA Rafinesque, 1815.............................. 178 Family ARISTEIDAE Wood-Mason & Alcock, 1891..................... 178 Family BENTHESICYMIDAE Wood-Mason & Alcock, 1891 .......... 180 Family PENAEIDAE Rafinesque, 1815 .................................. -

Experimental Studies on the Feeding Ecology of Munida Subrugosa (White, 1847) (Decapoda: Anomura: Galatheidae) from the Magellan Region, Southern Chile
SCIENTIA MARINA 71(1) March 2007, 187-190, Barcelona (Spain) ISSN: 0214-8358 Experimental studies on the feeding ecology of Munida subrugosa (White, 1847) (Decapoda: Anomura: Galatheidae) from the Magellan region, southern Chile PATRICIA KARAS1,2, MATTHIAS GORNY1 and RUBÉN ALARCÓN-MUÑOZ1 1 Instituto de la Patagonia, Universidad de Magallanes. Av. Bulnes 01890, Punta Arenas, Chile. E-mail: [email protected] 2 Institute for Polar Ecology, University of Kiel, D-24184 Kiel, Germany. SUMMARY: Feeding behaviour and food uptake rates of the anomuran crab Munida subrugosa from the southern Magellan region (Chile) were studied under laboratory conditions. Crabs exhibited a marked preference for meat versus macroalgae, with uptake rates being 14 times higher. Different algal species were ranked according to structure and detrital cover. Observations suggest that scavenging and cannibalism, restricted to injured or moulting individuals, are regularly applied in the natural habitat, whereas active hunting is only performed on suitable prey. The main food resource is suggested to be of microscopic origin, given the time spent on typical food uptake movements for detritus and suspended food. The findings of this study, backed up by results of simultaneously carried out stomach analysis, proved M. subrugosa to be an opportunistic feeder that can make use of different forms of food uptake. Key words: feeding habits, food choice, natural diet, anomuran crabs, Munida subrugosa. RESUMEN: ESTUDIOS EXPERIMENTALES DE LA ECOLOGÍA ALIMENTARIA DE MUNIDA SUBRUGOSA (WHITE, 1847) (DECAPODA: ANOMURA: GALATHEIDAE) DE LA REGIÓN DE MAGALLANES, SUR DE CHILE. – Se estudiaron los hábitos alimentarios y tasas de ingesta de alimento del crustáceo anomuro Munida subrugosa en la Región de Magallanes (Chile) bajo condiciones de labo- ratorio. -
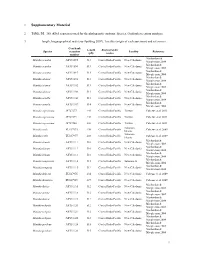
Supplementary Material
1 Supplementary Material 2 Table S1. 16S rRNA sequences used for the phylogenetic analysis. Species, Genbank accession numbers, 3 length, biogeographical reals (see Spalding 2007), Locality (origin of each specimen) and references. Genebank Length Biogeographic Species accession Locality Reference (pb) realms number Machordom & Munida acantha AY351095 513 Central Indo-Pacific New Caledonia Macpherson 2004 Machordon & Munida acantha AY351096 513 Central Indo-Pacific New Caledonia Macpherson 2004 Machordon & Munida acantha AY351097 513 Central Indo-Pacific New Caledonia Macpherson 2004 Machordon & Munida alonsoi AY351098 513 Central Indo-Pacific New Caledonia Macpherson 2004 Machordon & Munida alonsoi AY351102 513 Central Indo-Pacific New Caledonia Macpherson 2004 Machordon & Munida alonsoi AY351104 513 Central Indo-Pacific New Caledonia Macpherson 2004 Machordon & Munida armilla AY351106 513 Central Indo-Pacific New Caledonia Macpherson 2004 Machordon & Munida armilla AY351107 514 Central Indo-Pacific New Caledonia Macpherson 2004 Munida asprosoma JF727277 446 Central Indo-Pacific Taiwan Cabezas et al. 2011 Munida asprosoma JF727279 446 Central Indo-Pacific Taiwan Cabezas et al. 2011 Munida asprosoma JF727280 446 Central Indo-Pacific Taiwan Cabezas et al. 2011 Solomon Munida caeli EU417975 430 Central Indo-Pacific Cabezas et al. 2009 Islands Solomon Munida caeli EU417977 430 Central Indo-Pacific Cabezas et al. 2009 Islands Machordon & Munida clinata AY351111 516 Central Indo-Pacific New Caledonia Macpherson 2004 Machordon & Munida clinata AY351112 516 Central Indo-Pacific New Caledonia Macpherson 2004 Machordon & Munida clinata AY351113 516 Central Indo-Pacific New Caledonia Macpherson 2004 Machordon & Munida compressa AY351114 513 Central Indo-Pacific Salomon Is. Macpherson 2004 Machordon & Munida congesta AY351115 512 Central Indo-Pacific New Caledonia Macpherson 2004 Munida delicata EU417976 434 Central Indo-Pacific New Caledonia Cabezas et al. -

(Decapoda: Anomura: Galatheoidea) from the Mesozoic, Restriction of the Genus Paragalathea, and Establishment of 6 New Genera and 20 New Species
Ann. Naturhist. Mus. Wien, Serie A 118 65–131 Wien, 15 Jan. 2016 New families Paragalatheidae and Catillogalatheidae (Decapoda: Anomura: Galatheoidea) from the Mesozoic, restriction of the genus Paragalathea, and establishment of 6 new genera and 20 new species Cristina M. ROBINS1, Rodney M. FELDMANN2, Carrie E. SCHWEITZER3 & Aubrey BONDE4 (With 18 figures and 7 tables) Manuscript submitted on October 1st 2014, the revised manuscript on September 30th 2015. Abstract Two new families of Galatheoidea from the Mesozoic are described. Paragalatheidae encom- passes members of Paragalathea and Mesogalathea, as well as two new genera: Discutiolira and Lemacola. The genus Paragalathea is herein restricted to its original definition. New species within the Paragalatheidae established in this paper are: Paragalathea arcella, P. crenarvina, P. ternata, P. vultuosona, Lemacola jenniferae, L. rossi, L. salia, Mesogalathea macra, M. pyxis, and M. retusa. The new family Catillogalatheidae is described to encompass species from the Late Jurassic and Cretaceous. New genera established within Catillogalatheidae include: Catil logalathea, Tuberosagalathea, Serraphylctaena, and Vasconilia. New species established within Catillogalatheidae are: Catillogalathea falcula, Catillogalathea patruliusi, Catillogalathea pur carenensis, Galatheites aiola, G. diasema, G. obtecta, Hispanigalathea tithonia, Tuberosagala thea antefixa, T. tornatilis, and Vasconilia xystosa. The genus Galatheites is redescribed. Some observations and remarks are made on the preservation -

Deep-Sea Life Issue 16, January 2021 Cruise News Sedimentation Effects Survey Series (ROBES III) Completed
Deep-Sea Life Issue 16, January 2021 Despite the calamity caused by the global pandemic, we are pleased to report that our deep ocean continues to be investigated at an impressive rate. Deep-Sea Life 16 is another bumper issue, brimming with newly published research, project news, cruise news, scientist profiles and so on. Even though DOSI produce a weekly Deep-Sea Round Up newsletter and DOSI and DSBS are active on social media, there’s still plenty of breaking news for Deep- Sea Life! Firstly a quick update on the status of INDEEP. As most of you are aware, INDEEP was a legacy programme of the Census of Marine Life (2000-2010) and was established to address knowledge gaps in deep-sea ecology. Among other things, the INDEEP project played central role in the creation of the Deep-Ocean Stewardship Initiative and funded initial DOSI activities. In 2018, the DOSI Decade of Ocean Science working group was established with a view to identifying key priorities for deep-ocean science to support sustainable development and to ensure deep- ocean ecological studies were included in the UN Decade plans via truly global collaborative science. This has resulted in an exciting new initiative called “Challenger 150”. You are all invited to learn more about this during a webinar on 9th Feb (see p. 22 ). INDEEP has passed on the baton and has now officially closed its doors.Eva and I want to sincerely thank all those that led INDEEP with us and engaged in any of the many INDEEP actions. It was a productive programme that has left a strong legacy. -

From Korean Waters Jung Nyun Kim1*, Mi Hyang Kim2, Jung Hwa Choi3 and Yang Jae Im1
Kim et al. Fisheries and Aquatic Sciences (2016) 19:34 DOI 10.1186/s41240-016-0034-8 RESEARCH ARTICLE Open Access Galatheoid squat lobsters (Crustacea: Decapoda: Anomura) from Korean waters Jung Nyun Kim1*, Mi Hyang Kim2, Jung Hwa Choi3 and Yang Jae Im1 Abstract Ten species of Galatheoidea (squat lobsters), belonging to two families, were collected in the Korean exclusive economic zone: Galathea balssi Miyake and Baba, 1964, Galathea orientalis Stimpson, 1858, Galathea pubescens Stimpson, 1858, and Galathea rubromaculata Miyake and Baba, 1967 belonging to Galatheidae; Bathymunida brevirostris Yokoya, 1933, Cervimunida princeps Benedict, 1902, Munida caesura Macpherson and Baba, 1993, Munida japonica Stimpson, 1858, Munida pherusa Macpherson and Baba, 1993, and Paramunida scabra (Henderson, 1885) belonging to Munididae. The present study comprises the morphological description of these ten species, including drawings and color photographs, a brief review of their regional records, and a key for their identification. Although all species are common in Japanese waters, G. balssi, G. rubromaculata, B. brevirostris, C. princeps, M. caesura, and M. pherusa are new to Korean marine fauna. Keywords: Galatheidae, Munididae, Squat lobster, Korean fauna Background scabra (Henderson, 1885) (as Munida) in Munididae (as Based on a phylogenetic study, Ahyong et al. (2010) re- Galatheidae). vised the classification of the superfamily Galatheoidea The present study provides morphological descriptions to include four families: Galatheidae, Munididae, Muni- and illustrations for ten species of squat lobsters (four dopsidae, and Porcellanidae. The former three families, Galatheidae and six Munididae), collected in the Korean together with the superfamily Chirostyloidea, which in- exclusive economic zone during a fisheries resources in- cludes Chirostylidae, Eumunidae, and Kiwaidae, are vestigation conducted by the National Institute of known as “squat lobsters,” as their abdomen is tucked Fisheries Science (Korea), from 2003 to 2013. -

Ecology of Munida Gregaria (Decapoda, Anomura): Distribution and Abundance, Population Dynamics and Fisheries
MARINE ECOLOGY PROGRESS SERIES Vol. 22: 77-99. 1985 - Published February 28 Mar. Ecol. Prog. Ser. Ecology of Munida gregaria (Decapoda, Anomura): distribution and abundance, population dynamics and fisheries John R. Zeldis* Portobello Marine Laboratory, University of Otago. Dunedin, New Zealand ABSTRACT: Pelagic larvae, postlarvae and benthic adults of the galatheid crab Munida gregaria (Fabricius 1793) occur along the continental shelf of the east coast of the South Island and around the subantarctic islands of New Zealand. In the south-eastern South Island, larvae appear in June or July and develop through 5 zoeal stages. As they age, the larvae accumulate inshore and north of the Otago Peninsula. Following metamorphosis in October, the pelagic postlarvae shoal through the summer prior to settlement to the bottom. The length of the shoaling period can vary considerably from year to year, ranging from a few weeks to 6 mo or longer. The pelagic postlarvae are very patchy in spatial distribution. Postlarval biomass, as determined by aerial surveys along the south-east coast, was highest along the inner to middle shelf from Blueskin Bay to Moeraki, immediately north of the Otago Peninsula. Benthic settlement was also heavier in this area relative to south of the Peninsula. This provides evidence that a meso-scale eddy interrupts the northward drift of larvae and postlarvae in the Southland Current and retains them near the upstream boundary of the benthic population. In the Otago Peninsula area substantial benthic recruitment occurred only when and where the density of older cohorts on the bottom was low. After relatively long shoaling periods the 197&1978 cohorts settled on inner shelf sands and migrated to middle and outer shelf bryozoan-covered bottoms within a few months. -

Tapa TESIS M-VARISCO
Naturalis Repositorio Institucional Universidad Nacional de La Plata http://naturalis.fcnym.unlp.edu.ar Facultad de Ciencias Naturales y Museo Biología de Munida gregaria (Crustacea Anomura) : bases para su aprovechamiento pesquero en el Golfo San Jorge, Argentina Varisco, Martín Alejandro Doctor en Ciencias Naturales Dirección: Lopretto, Estela Celia Co-dirección: Vinuesa, Julio Héctor Facultad de Ciencias Naturales y Museo 2013 Acceso en: http://naturalis.fcnym.unlp.edu.ar/id/20130827001277 Esta obra está bajo una Licencia Creative Commons Atribución-NoComercial-CompartirIgual 4.0 Internacional Powered by TCPDF (www.tcpdf.org) Universidad Nacional de la Plata Facultad de Ciencias Naturales y Museo Tesis Doctoral Biología de Munida gregaria (Crustacea Anomura): bases para su aprovechamiento pesquero en el Golfo San Jorge, Argentina Lic. Martín Alejandro Varisco Directora Dra. Estela C. Lopretto Co-director Dr. Julio H. Vinuesa La Plata 2013 Esta tesis esta especialmente dedicada a mis padres A Evangelina A Pame, Jime, Panchi y Agus Agradecimientos Deseo expresar mi conocimiento a aquellas personas e instituciones que colaboraron para que llevar adelante esta tesis y a aquellos que me acompañaron durante la carrera de doctorado: Al Dr. Julio Vinuesa, por su apoyo constante y por su invaluable aporte a esta tesis en particular y a mi formación en general. Le agradezco también por permitirme trabajar con comodidad y por su apoyo cotidiano. A la Dra. Estela Lopretto, por su valiosa dedicación y contribución en esta Tesis. A los Lic. Héctor Zaixso y Damián Gil, por la colaboración en los análisis estadísticos A mis compañeros de trabajo: Damián, Paula, Mauro, Tomas, Héctor por su colaboración, interés y consejo en distintas etapas de este trabajo; pero sobre todo por hacer ameno el trabajo diario. -

Reappraisal of Anomuran and Brachyuran Decapods from the Lower Miocene Morozaki Group, Japan, Collected by the Tokai Fossil Soci
Bulletin of the Mizunami Fossil Museum, no. 43 (2017), p. 47–69, 1 fig., 13 pls. © 2017, Mizunami Fossil Museum Reappraisal of anomuran and brachyuran decapods from the lower Miocene Morozaki Group, Japan, collected by the Tokai Fossil Society Hiroaki Karasawa*, Yoshiaki Mizuno**, Kiichiro Hachiya**, and Yusuke Ando* *Mizunami Fossil Museum, Yamanouchi, Akeyo, Mizunami, Gifu 509-6132, Japan <[email protected]> ** Tokai Fossil Society, Sawashita 9-21, Atsuta, Nagoya, Aichi 456-0006, Japan Abstract A collection of anomuran and brachyuran decapods from the lower Miocene Morozaki Group of Aichi Prefecture, obtained by the Tokai Fossil Society, has led to revisions of previously known and unrecorded species. Mizunotengus makiguchimai Karasawa and Ando, a new genus and species of Munidopsidae is described. Mizunotengus is the secondary known extinct genus within the Miocene munidopsids. Paralithodes bishuensis Karasawa and Ando, a new species of Lithodidae, represents the first record for the genus. Munididae, Dicranodromia sp. (Homolodromidae), Hyastenus sp. (Epialtidae), Pisoides? sp. (Epialtidae), and Hexapinus sp. (Hexapodidae) are recognized. A remarkable preservation of Mizunotengus makiguchimai is briefly discussed. Key words: Decapoda, Anomura, Brachyura, Miocene, Morozaki Group, Aichi, Japan Introduction Miocene (the middle to upper part of Crucidenticula sawamurae Zone of Diatom zonation by Yanagisawa and The fossil marine pancrustaceans were collected from the Akiba (1998)) (Ito et al., 1999). lower Miocene Morozaki Group in Aichi Prefecture, Japan, The specimens described here are deposited in the during the field survey of the Tokai Fossil Society in 1983– Mizunami Fossil Museum (MFM). 1991. Takeda, Mizuno, and Yamaoka (1986) examined these specimens and described a thoracican Lepas sp., an amphipod Megaceradocus sp.