High Levels of Realized Dispersal and Phenotypic Plasticity in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evidence for Cospeciation Events in the Host–Symbiont System Involving Crinoids (Echinodermata) and Their Obligate Associates, the Myzostomids (Myzostomida, Annelida)
Molecular Phylogenetics and Evolution 54 (2010) 357–371 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Evidence for cospeciation events in the host–symbiont system involving crinoids (Echinodermata) and their obligate associates, the myzostomids (Myzostomida, Annelida) Déborah Lanterbecq a,*, Grey W. Rouse b, Igor Eeckhaut a a Marine Biology Laboratory, University of Mons, 6 Av. du Champ de Mars, Bât. Sciences de la vie, B-7000 Mons, Belgium b Scripps Institution of Oceanography, University of California, San Diego, La Jolla, CA 92093-0202, USA article info abstract Article history: Although molecular-based phylogenetic studies of hosts and their associates are increasingly common Received 14 April 2009 in the literature, no study to date has examined the hypothesis of coevolutionary process between Revised 3 August 2009 hosts and commensals in the marine environment. The present work investigates the phylogenetic Accepted 12 August 2009 relationships among 16 species of obligate symbiont marine worms (Myzostomida) and their echino- Available online 15 August 2009 derm hosts (Crinoidea) in order to estimate the phylogenetic congruence existing between the two lin- eages. The combination of a high species diversity in myzostomids, their host specificity, their wide Keywords: variety of lifestyles and body shapes, and millions years of association, raises many questions about Coevolution the underlying mechanisms triggering their diversification. The phylogenetic -

Squat Lobsters of the Genus Munida (Crustacea: Decapoda: Anomura: Munididae) from the Ogasawara Islands, with Descriptions of Four New Species
国立科博専報,(47): 339–365,2011年4月15日 Mem. Natl. Mus. Nat. Sci., Tokyo, (47): 339–365, April 15, 2011 Squat Lobsters of the Genus Munida (Crustacea: Decapoda: Anomura: Munididae) from the Ogasawara Islands, with Descriptions of Four New Species Tomoyuki Komai Natural History Museum and Institute, Chiba, 955–2 Aoba-cho, Chuo-ku, Chiba-shi, Chiba 260–8682, Japan E-mail: [email protected] Abstract. The present study reports on the squat lobster genus Munida Leach, 1820 (Anomura: Munididae) collected in the Ogasawara Islands during the Project “Studies on the Origin of Bio- diversity in the Sagami Sea Fossa Magna Element and the Izu-Ogasawara (Bonin) Arc” in 2006–2010, carried out by the National Museum and Nature and Science. Six species were iden- tified, including four new species: M. disiunctus sp. nov., M. honshuensis Benedict, 1902, M. koyo sp. nov., M. longinquus sp. nov., M. munin sp. nov., and M pectinata Macpherson and Ma- chordom, 2005. The two previously described species are newly recorded from the area, of them M. pectinata is first recorded from waters outside New Caledonia. Affinities of the four new spe- cies are discussed. Key words: Crustacea, Munididae, Munida, new species, Ogasawara Islands Pacific (e.g., Baba, 1988; 1994; 2005; Baba et al., Introduction 2009; Macpherson, 1993; 1994; 1996a; 1996b; The galatheoid fauna of the oceanic Ogasawara 1997; 1999a; 1999b; 2000; 2004; 2006a; 2006b; Islands, located at about 1000 km south of Tokyo, 2009; Macpherson and de Saint Laurent, 1991; central Japan, is little known, although some pub- Macpherson and Baba, 1993; Macpherson and lications have been published (Stimpson, 1858; Machordom, 2005; Machordom and Macpherson, Balss, 1913; Melin, 1939; Miyake and Baba, 2004; Ahyong and Poore, 2004; Ahyong, 2007). -

From Ghost and Mud Shrimp
Zootaxa 4365 (3): 251–301 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2017 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4365.3.1 http://zoobank.org/urn:lsid:zoobank.org:pub:C5AC71E8-2F60-448E-B50D-22B61AC11E6A Parasites (Isopoda: Epicaridea and Nematoda) from ghost and mud shrimp (Decapoda: Axiidea and Gebiidea) with descriptions of a new genus and a new species of bopyrid isopod and clarification of Pseudione Kossmann, 1881 CHRISTOPHER B. BOYKO1,4, JASON D. WILLIAMS2 & JEFFREY D. SHIELDS3 1Division of Invertebrate Zoology, American Museum of Natural History, Central Park West @ 79th St., New York, New York 10024, U.S.A. E-mail: [email protected] 2Department of Biology, Hofstra University, Hempstead, New York 11549, U.S.A. E-mail: [email protected] 3Department of Aquatic Health Sciences, Virginia Institute of Marine Science, College of William & Mary, P.O. Box 1346, Gloucester Point, Virginia 23062, U.S.A. E-mail: [email protected] 4Corresponding author Table of contents Abstract . 252 Introduction . 252 Methods and materials . 253 Taxonomy . 253 Isopoda Latreille, 1817 . 253 Bopyroidea Rafinesque, 1815 . 253 Ionidae H. Milne Edwards, 1840. 253 Ione Latreille, 1818 . 253 Ione cornuta Bate, 1864 . 254 Ione thompsoni Richardson, 1904. 255 Ione thoracica (Montagu, 1808) . 256 Bopyridae Rafinesque, 1815 . 260 Pseudioninae Codreanu, 1967 . 260 Acrobelione Bourdon, 1981. 260 Acrobelione halimedae n. sp. 260 Key to females of species of Acrobelione Bourdon, 1981 . 262 Gyge Cornalia & Panceri, 1861. 262 Gyge branchialis Cornalia & Panceri, 1861 . 262 Gyge ovalis (Shiino, 1939) . 264 Ionella Bonnier, 1900 . -
A New Species of Squat Lobster of the Genus Hendersonida (Crustacea, Decapoda, Munididae) from Papua New Guinea
ZooKeys 935: 25–35 (2020) A peer-reviewed open-access journal doi: 10.3897/zookeys.935.51931 RESEARCH ARTICLE https://zookeys.pensoft.net Launched to accelerate biodiversity research A new species of squat lobster of the genus Hendersonida (Crustacea, Decapoda, Munididae) from Papua New Guinea Paula C. Rodríguez-Flores1,2, Enrique Macpherson1, Annie Machordom2 1 Centre d’Estudis Avançats de Blanes (CEAB-CSIC), C. acc. Cala Sant Francesc 14 17300 Blanes, Girona, Spain 2 Museo Nacional de Ciencias Naturales (MNCN-CSIC), José Gutiérrez Abascal, 2, 28006 Madrid, Spain Corresponding author: Paula C. Rodríguez-Flores ([email protected]) Academic editor: I.S. Wehrtmann | Received 10 March 2020 | Accepted 2 April 2020 | Published 21 May 2020 http://zoobank.org/E2D29655-B671-4A4C-BCDA-9A8D6063D71D Citation: Rodríguez-Flores PC, Macpherson E, Machordom A (2020) A new species of squat lobster of the genus Hendersonida (Crustacea, Decapoda, Munididae) from Papua New Guinea. ZooKeys 935: 25–35. https://doi. org/10.3897/zookeys.935.51931 Abstract Hendersonida parvirostris sp. nov. is described from Papua New Guinea. The new species can be distin- guished from the only other species of the genus, H. granulata (Henderson, 1885), by the fewer spines on the dorsal carapace surface, the shape of the rostrum and supraocular spines, the antennal peduncles, and the length of the walking legs. Pairwise genetic distances estimated using the 16S rRNA and COI DNA gene fragments indicated high levels of sequence divergence between the new species and H. granulata. Phylogenetic analyses, however, recovered both species as sister species, supporting monophyly of the genus. Keywords Anomura, mitochondrial genes, morphology, West Pacific Introduction Squat lobsters of the family Munididae Ahyong, Baba, Macpherson & Poore, 2010 are recognised by the trispinose or trilobate front, usually composed of a slender rostrum flanked by supraorbital spines (Ahyong et al. -

Deep-Water Squat Lobsters (Crustacea: Decapoda: Anomura) from India Collected by the FORV Sagar Sampada
Bull. Natl. Mus. Nat. Sci., Ser. A, 46(4), pp. 155–182, November 20, 2020 Deep-water Squat Lobsters (Crustacea: Decapoda: Anomura) from India Collected by the FORV Sagar Sampada Vinay P. Padate1, 2, Shivam Tiwari1, 3, Sherine Sonia Cubelio1,4 and Masatsune Takeda5 1Centre for Marine Living Resources and Ecology, Ministry of Earth Sciences, Government of India. Atal Bhavan, LNG Terminus Road, Puthuvype, Kochi 682508, India 2Corresponding author: [email protected]; https://orcid.org/0000-0002-2244-8338 [email protected]; https://orcid.org/0000-0001-6194-8960 [email protected]; http://orcid.org/0000-0002-2960-7055 5Department of Zoology, National Museum of Nature and Science, Tokyo. 4–1–1 Amakubo, Tsukuba, Ibaraki 305–0005, Japan. [email protected]; https://orcid/org/0000-0002-0028-1397 (Received 13 August 2020; accepted 23 September 2020) Abstract Deep-water squat lobsters collected during five cruises of the Fishery Oceanographic Research Vessel Sagar Sampada off the Andaman and Nicobar Archipelagos (299–812 m deep) and three cruises in the southeastern Arabian Sea (610–957 m deep) are identified. They are referred to each one species of the families Chirostylidae and Sternostylidae in the Superfamily Chirostyloidea, and five species of the family Munidopsidae and three species of the family Muni- didae in the Superfamily Galatheoidea. Of altogether 10 species of 5 genera dealt herein, the Uro- ptychus species of the Chirostylidae is described as new to science, and Agononida aff. indocerta Poore and Andreakis, 2012, of the Munididae, previously reported from Western Australia and Papua New Guinea, is newly recorded from Indian waters. -

Annotated Checklist of New Zealand Decapoda (Arthropoda: Crustacea)
Tuhinga 22: 171–272 Copyright © Museum of New Zealand Te Papa Tongarewa (2011) Annotated checklist of New Zealand Decapoda (Arthropoda: Crustacea) John C. Yaldwyn† and W. Richard Webber* † Research Associate, Museum of New Zealand Te Papa Tongarewa. Deceased October 2005 * Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, New Zealand ([email protected]) (Manuscript completed for publication by second author) ABSTRACT: A checklist of the Recent Decapoda (shrimps, prawns, lobsters, crayfish and crabs) of the New Zealand region is given. It includes 488 named species in 90 families, with 153 (31%) of the species considered endemic. References to New Zealand records and other significant references are given for all species previously recorded from New Zealand. The location of New Zealand material is given for a number of species first recorded in the New Zealand Inventory of Biodiversity but with no further data. Information on geographical distribution, habitat range and, in some cases, depth range and colour are given for each species. KEYWORDS: Decapoda, New Zealand, checklist, annotated checklist, shrimp, prawn, lobster, crab. Contents Introduction Methods Checklist of New Zealand Decapoda Suborder DENDROBRANCHIATA Bate, 1888 ..................................... 178 Superfamily PENAEOIDEA Rafinesque, 1815.............................. 178 Family ARISTEIDAE Wood-Mason & Alcock, 1891..................... 178 Family BENTHESICYMIDAE Wood-Mason & Alcock, 1891 .......... 180 Family PENAEIDAE Rafinesque, 1815 .................................. -

Chemical Communication in Crustaceans
Chemical Communication in Crustaceans Thomas Breithaupt l Martin Thiel Editors Chemical Communication in Crustaceans With drawings by Jorge Andrés Varela Ramos Editors Thomas Breithaupt Martin Thiel University of Hull Universidad Cato´lica del Norte Department of Biological Sciences Facultad Ciencas del Mar Hull, HU6 7RX Depto. de Biologı´a Marina UK Larrondo 1281 [email protected] Coquimbo Chile [email protected] ISBN 978-0-387-77100-7 e-ISBN 978-0-387-77101-4 DOI 10.1007/978-0-387-77101-4 Springer New York Dordrecht Heidelberg London # Springer Science+Business Media, LLC 2011 All rights reserved. This work may not be translated or copied in whole or in part without the written permission of the publisher (Springer Science+Business Media, LLC, 233 Spring Street, New York, NY 10013, USA), except for brief excerpts in connection with reviews or scholarly analysis. Use in connection with any form of information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed is forbidden. The use in this publication of trade names, trademarks, service marks, and similar terms, even if they are not identified as such, is not to be taken as an expression of opinion as to whether or not they are subject to proprietary rights. Printed on acid-free paper Springer is part of Springer ScienceþBusiness Media (www.springer.com) To our families and friends. Preface Animal communication has fascinated biologists for centuries. This fascination has sustained many a scientific career as will be evident from the personal accounts by the contributors to this book. -

Experimental Studies on the Feeding Ecology of Munida Subrugosa (White, 1847) (Decapoda: Anomura: Galatheidae) from the Magellan Region, Southern Chile
SCIENTIA MARINA 71(1) March 2007, 187-190, Barcelona (Spain) ISSN: 0214-8358 Experimental studies on the feeding ecology of Munida subrugosa (White, 1847) (Decapoda: Anomura: Galatheidae) from the Magellan region, southern Chile PATRICIA KARAS1,2, MATTHIAS GORNY1 and RUBÉN ALARCÓN-MUÑOZ1 1 Instituto de la Patagonia, Universidad de Magallanes. Av. Bulnes 01890, Punta Arenas, Chile. E-mail: [email protected] 2 Institute for Polar Ecology, University of Kiel, D-24184 Kiel, Germany. SUMMARY: Feeding behaviour and food uptake rates of the anomuran crab Munida subrugosa from the southern Magellan region (Chile) were studied under laboratory conditions. Crabs exhibited a marked preference for meat versus macroalgae, with uptake rates being 14 times higher. Different algal species were ranked according to structure and detrital cover. Observations suggest that scavenging and cannibalism, restricted to injured or moulting individuals, are regularly applied in the natural habitat, whereas active hunting is only performed on suitable prey. The main food resource is suggested to be of microscopic origin, given the time spent on typical food uptake movements for detritus and suspended food. The findings of this study, backed up by results of simultaneously carried out stomach analysis, proved M. subrugosa to be an opportunistic feeder that can make use of different forms of food uptake. Key words: feeding habits, food choice, natural diet, anomuran crabs, Munida subrugosa. RESUMEN: ESTUDIOS EXPERIMENTALES DE LA ECOLOGÍA ALIMENTARIA DE MUNIDA SUBRUGOSA (WHITE, 1847) (DECAPODA: ANOMURA: GALATHEIDAE) DE LA REGIÓN DE MAGALLANES, SUR DE CHILE. – Se estudiaron los hábitos alimentarios y tasas de ingesta de alimento del crustáceo anomuro Munida subrugosa en la Región de Magallanes (Chile) bajo condiciones de labo- ratorio. -

This Article Appeared in a Journal Published by Elsevier. the Attached
(This is a sample cover image for this issue. The actual cover is not yet available at this time.) This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Fisheries Research 129–130 (2012) 28–37 Contents lists available at SciVerse ScienceDirect Fisheries Research jou rnal homepage: www.elsevier.com/locate/fishres Annual brood number and breeding periodicity of squat lobsters (Decapoda: Anomura: Galatheidae) from the continental shelf of the SE Pacific—Implications for fisheries management a,b,∗ a a b Martin Thiel , Nuxia L. Espinoza-Fuenzalida , Enzo Acuna˜ , Marcelo M. Rivadeneira a Facultad Ciencias del Mar, Universidad Católica del Norte, Larrondo 1281, Coquimbo, Chile b Centro de Estudios Avanzados en Zonas Áridas, CEAZA, Coquimbo, Chile a r t i c l e i n f o a b s t r a c t Article history: The reproductive potential of a population depends on the number of broods that individuals produce Received 3 January 2012 during the annual reproductive season. -

Population Dynamics and Fisheries of Squat Lobsters, Family Galatheidae, in Chile
Population Dynamics and Fisheries of Squat Lobsters, Family Galatheidae, in Chile N. Bahamonde Unlversidad de Child, Facuitad de Cienclas, Departamento de Cienolaa Ecologlpas, Casilla 653, Santiago, Chile G. Henrfquez Institute de Fomento Pesquero, IFOR, Divisidn de Recursos, Casilla 1287, Santiago, Chile A. Zuleta Subsecreiarfa de Pesea, Mlnisterlo de Economfa, Fomento y Reconstruccidn, Teatinos 120, Santiago, Chile and H. Bustos and R. Bahamonde Instituto de Fomento Pesquero, IFOP, Division de Recursos, Casilla 128?, Santiago, Chile RAHAMONOE, N., C. HkNRlQUCZ, A. ZULETA, H. BUSTOS, AND K. BAHAMONDE. 1966. Population dynamics and fisheries of squat lobsters, family Galatheidae, in Child, p. 254-268. In G. S. Jamieson and N. Bourne fed.] North Pacific Workshop on stock assessment and management of invertebrates. Can. Spec. Publ. Fish. Aquat. Sci.92. Two species of squat lobsters, Cerv/mumdct johni. Porter, and Pleuroncodes monodon, Milne Edwards, family Galatheidae. are harvested commercially off Chile. A commercial fishery began by exploiting the first spe cies bur. has gradually shifted to harvesting the second. The fishery for Pleuroncodes began in 1966 off San Antonio {L. 33°3S"SJ but has gradually moved southward and now extends to Talcahuano (37°S). There are two possible explanations for this shift in fishing area; (1) increased fishing pressure, and (2) changes in the natural environment. Some support for the second explanation is seen by simultaneous changes observed in other species which may be due to cyclic changes in the environment. Data on the fishery has been collected from 1%b to the present from the area between Coquimbo (30°S) and Talcahuano by the Fisheries Development Institute of Chile (Instituto de Fomento Pesquero, IFOP) by monitoring the fleet at landing ports and from research surveys. -

Turbo-Taxonomy: 21 New Species of Myzostomida (Annelida)
Zootaxa 3873 (4): 301–344 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2014 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3873.4.1 http://zoobank.org/urn:lsid:zoobank.org:pub:84F8465A-595F-4C16-841E-1A345DF67AC8 Turbo-taxonomy: 21 new species of Myzostomida (Annelida) MINDI M. SUMMERS1,3, IIN INAYAT AL-HAKIM2 & GREG W. ROUSE1,3 1Scripps Institution of Oceanography, UCSD, 9500 Gilman Drive, La Jolla, CA 92093, USA 2Research Center for Oceanography, Indonesian Institute of Sciences (RCO-LIPI), Jl. Pasir Putih I, Ancol Timur, Jakarta 14430, Indonesia 3Correspondence. E-mail: [email protected]; [email protected] Abstract An efficient protocol to identify and describe species of Myzostomida is outlined and demonstrated. This taxonomic ap- proach relies on careful identification (facilitated by an included comprehensive table of available names with relevant geographical and host information) and concise descriptions combined with DNA sequencing, live photography, and ac- curate host identification. Twenty-one new species are described following these guidelines: Asteromyzostomum grygieri n. sp., Endomyzostoma scotia n. sp., Endomyzostoma neridae n. sp., Mesomyzostoma lanterbecqae n. sp., Hypomyzos- toma jasoni n. sp., Hypomyzostoma jonathoni n. sp., Myzostoma debiae n. sp., Myzostoma eeckhauti n. sp., Myzostoma hollandi n. sp., Myzostoma indocuniculus n. sp., Myzostoma josefinae n. sp., Myzostoma kymae n. sp., Myzostoma lau- renae n. sp., Myzostoma miki n. sp., Myzostoma pipkini n. sp., Myzostoma susanae n. sp., Myzostoma tertiusi n. sp., Pro- tomyzostomum lingua n. sp., Protomyzostomum roseus n. sp., Pulvinomyzostomum inaki n. sp., and Pulvinomyzostomum messingi n. sp. Key words: systematics, polychaete, marine, symbiosis, crinoid, asteroid, ophiuroid, parasite, taxonomic impediment Background Estimations of Earth’s biodiversity vary by orders of magnitude (e.g. -
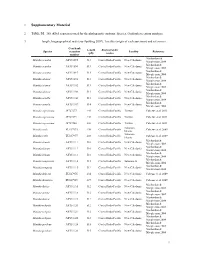
Supplementary Material
1 Supplementary Material 2 Table S1. 16S rRNA sequences used for the phylogenetic analysis. Species, Genbank accession numbers, 3 length, biogeographical reals (see Spalding 2007), Locality (origin of each specimen) and references. Genebank Length Biogeographic Species accession Locality Reference (pb) realms number Machordom & Munida acantha AY351095 513 Central Indo-Pacific New Caledonia Macpherson 2004 Machordon & Munida acantha AY351096 513 Central Indo-Pacific New Caledonia Macpherson 2004 Machordon & Munida acantha AY351097 513 Central Indo-Pacific New Caledonia Macpherson 2004 Machordon & Munida alonsoi AY351098 513 Central Indo-Pacific New Caledonia Macpherson 2004 Machordon & Munida alonsoi AY351102 513 Central Indo-Pacific New Caledonia Macpherson 2004 Machordon & Munida alonsoi AY351104 513 Central Indo-Pacific New Caledonia Macpherson 2004 Machordon & Munida armilla AY351106 513 Central Indo-Pacific New Caledonia Macpherson 2004 Machordon & Munida armilla AY351107 514 Central Indo-Pacific New Caledonia Macpherson 2004 Munida asprosoma JF727277 446 Central Indo-Pacific Taiwan Cabezas et al. 2011 Munida asprosoma JF727279 446 Central Indo-Pacific Taiwan Cabezas et al. 2011 Munida asprosoma JF727280 446 Central Indo-Pacific Taiwan Cabezas et al. 2011 Solomon Munida caeli EU417975 430 Central Indo-Pacific Cabezas et al. 2009 Islands Solomon Munida caeli EU417977 430 Central Indo-Pacific Cabezas et al. 2009 Islands Machordon & Munida clinata AY351111 516 Central Indo-Pacific New Caledonia Macpherson 2004 Machordon & Munida clinata AY351112 516 Central Indo-Pacific New Caledonia Macpherson 2004 Machordon & Munida clinata AY351113 516 Central Indo-Pacific New Caledonia Macpherson 2004 Machordon & Munida compressa AY351114 513 Central Indo-Pacific Salomon Is. Macpherson 2004 Machordon & Munida congesta AY351115 512 Central Indo-Pacific New Caledonia Macpherson 2004 Munida delicata EU417976 434 Central Indo-Pacific New Caledonia Cabezas et al.