Building a Fire
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

TD-1464S Publication Date: 7/20/2021 Rev: 3
Based on Standard: TD-1464S Publication Date: 7/20/2021 Rev: 3 Wildfire Prevention Contract Requirements SUMMARY PG&E’s standard establishes precautions for PG&E employees, PG&E suppliers, contractors, and third-party employees to follow when traveling to, performing work, or operating outdoors on any forest, brush, or grass-covered land. The information in this document is based on PG&E’s TD 1464s standard and local, state, and federal fire regulations and permits. However, if a local or state fire regulation or permit contains provisions more stringent than those in this document, the more stringent provisions must be followed. TARGET AUDIENCE This "based on TD-1464s" document targets all contractors performing work on behalf of PG&E and working on or near facilities located in any forest, brush, or grass-covered lands, using equipment, tools, and/or vehicles whose use could result in the ignition of a fire. This includes those areas that may seem urban but have vegetation that can aid in the spread of an ignition. TABLE OF CONTENTS Section Title Page 1 Safety ................................................................................................................ 1 2 General Requirements ...................................................................................... 2 3 Fire Index Process ............................................................................................ 7 4 Mitigations ......................................................................................................... 8 5 Quality Reviews ................................................................................................ 9 REQUIREMENTS 1 Safety 1.1 Performing utility work on any forest, brush, or grass-covered lands presents a danger of fire, in addition to the hazards inherent to utility work. 1.2 Following the directives in this standard are essential to mitigating fire danger and protecting the environment, the utility system, personnel, and the public. PG&E Internal ©2021 Pacific Gas and Electric Company. -

Fire Safety Trailer Curriculum
U.S. Fire Administration Acknowledgements Preparation of this Fire Safety Trailer Curriculum was made possible thanks to the cooperation and hard work of numerous firefighters, public information officers, public education coordinators, and staff of local fire departments and state fire marshal’s offices throughout the United States who contributed countless hours to review, test, and critique this curriculum. The results of their feedback and dedication will help fire safety professionals nationwide develop fire safety education and prevention programs designed to reduce fire-related injury and mortality rates suffered throughout the country. Development of the curriculum was funded by the Centers for Disease Control and Prevention (CDC), National Center for Injury Prevention and Control (NCIPC) under Contract No. 200-2007-21025 to Information Ventures, Inc. TABLE OF CONTENTS INTRODUCTION i–1 What’s in This Curriculum? i–1 How to Use This Curriculum i–2 Before Your Fire Safety Trailer Event i–2 During Your Fire Safety Trailer Event i–3 After Your Fire Safety Trailer Event i–4 1 HOW TO GET FUNDING FOR A TRAILER 1–1 Fire Prevention And Safety (FP&S) Grant 1–1 Grant Writing Tips 1–2 Getting Organized To Write Your Grant Application 1–4 Preparing Your Fire Prevention and Safety Grant Application 1–5 Preparing The Budget 1–7 Submitting Your Fire Prevention and Safety Application 1–8 Other Sources Of Grant Funding 1–9 Beyond The Basics 1–10 Grant Planning Guide 1–10 More Information 1–13 Resources 1–14 2 MARKETING AND COLLABORATING WITH SITES -

A History of the Prepare, Stay and Defend Or Leave Early Policy in Victoria
A History of the Prepare, Stay and Defend or Leave Early Policy in Victoria A thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy Benjamin Thomas Reynolds Master of Arts (History) Bachelor of Arts (History) School of Management College of Business RMIT University February 2017 1 Declaration I certify that except where due acknowledgement has been made, the work is that of the author alone; the work has not been submitted previously, in whole or in part, to qualify for any other academic award; the content of the thesis is the result of work which has been carried out since the official commencement date of the approved research program; any editorial work, paid or unpaid, carried out by a third party is acknowledged; and, ethics procedures and guidelines have been followed. Benjamin Thomas Reynolds February 2017 i Acknowledgements This PhD was made possible due to the support of my family, friends and supervisors and the guidance and encouragement I received from each. I would like to thank my parents in particular for again supporting me in my studies, and my supervisors Professor Peter Fairbrother, Dr Bernard Mees, and Dr Meagan Tyler and other colleagues in the School of Management for their reassurances, time, and advice. I would also like to thank the Bushfire and Natural Hazards Cooperative Research Centre for their generous financial support for the project, and in particular Annette Allen and Lyndsey Wright for their encouragement along the way. I would also like to acknowledge the support of John Schauble of Emergency Management Victoria, without whose support the thesis would not have been possible. -

In the Autumn 2011 Edition of the Quiver I Wrote an Article Touching on the Topic of Survival As It Applies to the Bowhunter
In the Autumn 2011 edition of The Quiver I wrote an article touching on the topic of survival as it applies to the bowhunter. In this article I want to talk about fire specifically and the different types of firestarters and techniques available. Fire is an important element in a survival situation as it provides heat for warmth, drying clothes or cooking as well as a psychological boost and if you’re hunting in a spot where you are one of the prey species it can keep predators away as well. There are many ways to start a fire; some ways relatively easy and some that would only be used as a last resort. There are pros and cons to most of these techniques. The most obvious tool for starting a fire is a match. While this is a great way to start a fire in your fireplace or fire pit I personally don’t like to carry matches in my pack or on my person. They are hard to keep dry and you are limited to one fire per match IF you can light a one match fire every time. It would be easy to run out of matches in a hurry as you are limited in how many you could reasonably carry. A Bic lighter or one of the more expensive windproof lighters is a slightly better choice for the bowhunter to carry. They are easy to use, easy to carry, fairly compact, and last for a reasonable amount of “lights”. They don’t work well when wet but can be dried out fairly easily. -
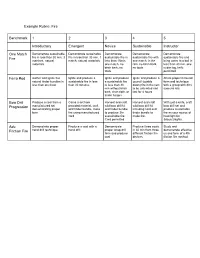
Example Rubric: Fire Benchmark 1 2 3 4 5 Introductory Emergent Novice
Example Rubric: Fire Benchmark 1 2 3 4 5 Introductory Emergent Novice Sustainable Instructor One Match Demonstrate sustainable Demonstrate sustainable Demonstrate Demonstrate Demonstrate Fire fire in less than 30 min, 3 fire in less than 30 min, 1 sustainable fire in sustainable fire with sustainable fire and matches, natural match, natural materials. less than 15min, one match, in the bring water to a boil in materials. one match, no rain, no birch bark, less than 30 min, one birch bark, no no tools. cedar log, knife tools permitted Ferro Rod Gather and ignite five Ignite and produce a Ignite and produce Ignite and produce a Share proper ferro rod natural tinder bundles in sustainable fire in less a sustainable fire council (upside form and technique less than one hour than 30 minutes in less than 30 down) fire in the rain with a group with 80% min without birch to be untended and success rate bark, char cloth, or last for 4 hours tinder fungus Bow Drill Produce a coal from a Carve a set from Harvest and craft Harvest and craft With just a knife, craft Progression manufactured set provided material, craft wild bow drill kit wild bow drill kit bow drill set and demonstrating proper wild tinder bundle, make and tinder bundle including cord and produce sustainable form fire using manufactured to produce 3hr tinder bundle to fire as your source of cord sustainable fire. make fire. heat/light for Cord permitted 3days/2nights. Adv. Demonstrate proper Produce a coal with a Demonstrate Produce three coals Study and Friction Fire hand drill technique hand drill proper strap drill in 30 min from three demonstrate effective form and produce differant friction fire us and form of a 4th coal devices friction fire method. -

FINAL REPORT Title: Multi-Scale Study of Ember Production and Transport Under Multiple Environmental and Fuel Conditions JFSP PROJECT ID:15-1-04-9
FINAL REPORT Title: Multi-Scale Study of Ember Production and Transport under Multiple Environmental and Fuel Conditions JFSP PROJECT ID:15-1-04-9 October 7, 2019 David L. Blunck, PhD Oregon State University Bret Butler, PhD US Forest Service John Bailey, PhD Oregon State University Natalie Wagenbrenner, PhD US Forest Service The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the opinions or policies of the U.S. Government. Mention of trade names or commercial products does not constitute their endorsement by the U.S. Government. - 1 - Table of Contents Abstract ....................................................................................................................................... 5 1. Overview and Objectives ................................................................................................... 6 2. Background ........................................................................................................................ 7 3. Materials and Methods ....................................................................................................... 9 4.1 Branch-scale Studies ........................................................................................................ 10 4.2 Tree-scale Studies ............................................................................................................ 12 4.2.1 Experimental Approach .......................................................................................... -

Protect Your Property from Wildfire Table of Contents
www.firesafemarin.org CALIFORNIA EDITION Protect Your Property from Wildfire Table of Contents 4 You Can Make a Difference • An Overview of this Guide: Reducing the Vulnerability of Your Home or Business • Managing Vegetation and Other Combustible Materials Around Your Home or Business ○ Defensible Space • Understanding Terms: The Role of Building Codes and Test Standards for Materials ○ California Building Code Chapter 7A Summary • Improving the Wildfire Resistance of Your Home or Business 9 Roof Covering • Things to Keep in Mind When Choosing a Class A Roof Covering • Tile and Other Roof Coverings with Gaps at the Edges • Skylights 12 Gutters 13 Vents: Under-Eave, Attic and Crawl Space (Foundation) 15 Windows and Doors 18 Decks, Patios and Porches 21 Siding 23 Fences 24 Chimneys, Burn Barrels and Open Debris Burning 25 Vegetative Fuels Treatments Away from Buildings 25 Creating Defensible Space • Identifying Fuels Management Zones • Defensible Space ○ Zone 1: 0-5 Feet (Near-Home Noncombustible Zone) ○ Zone 2: 5-30 Feet (Lean, Clean and Green Zone) ○ Zone 3: 30-100 Feet 30 Firewood, Leftover Materials and Combustible Materials 30 Plants 31 Yard and Garden Structures 32 Outbuildings, Fuel Tanks and Combustibles 33 Importance of Topography 34 Importance of Environmental Condition 34 Defensive Actions • If You are Trapped and Cannot Leave • External Water Spray System • Gel Coatings 37 Additional Resources for Vegetation/Plant Selections 4 PROTECT YOUR PROPERTY FROM WILDFIRE YOU CAN MAKE A DIFFERENCE Research and post-fire assessments have shown that property owners can protect their homes and businesses against wildfire by addressing three clear sources of vulnerability: materials and design features used in building the home or business, the landscaping vegetation located immediately adjacent to the home or business, and the general vegetation and other combustible materials and items on the property surrounding the home or business. -

Fire Spread on Ember-Ignited Decks CONSTRUCTION
WILDFIRE RESEARCH FACT SHEET Fire Spread on Ember-Ignited Decks CONSTRUCTION Wind-blown embers generated during wildfires are the single biggest hazard wildfires pose to RECOMMENDATIONS homes, and homeowners should never overlook the potential risk that an attached deck can IBHS research shows that, for medium create. Recent testing by the Insurance Institute for Business & Home Safety (IBHS) offers density softwood decking products (such as redwood and cedar), which can be important findings that can help minimize risk from wind-blown embers to decks. vulnerable to ignition from embers, the associated fire spread on the deck can be Nothing that can ignite should be stored under a deck. This action, along with development minimized by the following: of effective and well-maintained home ignition zones, will minimize the chance of all but a wind- blown ember exposure to your deck. An ignited deck can result, for example, in the ignition of combustible siding, or glass breakage in a sliding glass door. Increase the gap between ABOUT THE RESEARCH TESTS regardless of the deck board’s orientation deck boards from 1/8 inch IBHS’s tests evaluated how an ember-ignited (parallel or perpendicular). When deck 1. to 1/4 inch. fire on an attached deck can spread to the boards were perpendicular to the building, home, and yielded important guidance to the fire would spread in the gap between Fire spread in the gap between deck boards. minimize the chance of fire spread to the boards. The 1/8” gap between deck boards Note the small flame burned all the way to house. -

On the Evolution of Human Fire Use
ON THE EVOLUTION OF HUMAN FIRE USE by Christopher Hugh Parker A dissertation submitted to the faculty of The University of Utah in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Anthropology The University of Utah May 2015 Copyright © Christopher Hugh Parker 2015 All Rights Reserved The University of Utah Graduate School STATEMENT OF DISSERTATION APPROVAL The dissertation of Christopher Hugh Parker has been approved by the following supervisory committee members: Kristen Hawkes , Chair 04/22/2014 Date Approved James F. O’Connell , Member 04/23/2014 Date Approved Henry Harpending , Member 04/23/2014 Date Approved Andrea Brunelle , Member 04/23/2014 Date Approved Rebecca Bliege Bird , Member Date Approved and by Leslie A. Knapp , Chair/Dean of the Department/College/School of Anthropology and by David B. Kieda, Dean of The Graduate School. ABSTRACT Humans are unique in their capacity to create, control, and maintain fire. The evolutionary importance of this behavioral characteristic is widely recognized, but the steps by which members of our genus came to use fire and the timing of this behavioral adaptation remain largely unknown. These issues are, in part, addressed in the following pages, which are organized as three separate but interrelated papers. The first paper, entitled “Beyond Firestick Farming: The Effects of Aboriginal Burning on Economically Important Plant Foods in Australia’s Western Desert,” examines the effect of landscape burning techniques employed by Martu Aboriginal Australians on traditionally important plant foods in the arid Western Desert ecosystem. The questions of how and why the relationship between landscape burning and plant food exploitation evolved are also addressed and contextualized within prehistoric demographic changes indicated by regional archaeological data. -

ANTHROPOLOGICAL RECORDS Volume 25
ANTHROPOLOGICAL RECORDS Volume 25 ETHNOGRAPHIC NOTES ON THE SOUTHWESTERN POMO BY E. W. GIFFORD UNIVERSITY OF CALIFORNIA PRESS BERKELEY AND LOS ANGELES 1967 ETHNOGRAPHIC NOTES ON THE SOUTHWESTERN POMO ETHNOGRAPHIC NOTES ON THE SOUTHWESTERN POMO BY E. W. GIFFORD ANTHROPOLOGICAL RECORDS Volume 25 UNIVERSITY OF CALIFORNIA PUBLICATIONS ANTHROPOLOGICAL RECORDS Advisory Editors: M. A. Baumhoff, D. J. Crowley, C. J. Erasmus, T. D. McCown, C. W. Meighan, H. P. Phillips, M. G. Smith Volume 25 Approved for publication May 20, 1966 Issued May 29, 1967 Price, $1.50 University of California Press Berkeley and Los Angeles C alifornia Cambridge University Press London, England Manufactured in the United States of America CONTENTS Introduction ............................................ 1 The Southwestern Pomo in Russian Times: An Account by Kostromitonow ..1 Data Obtained from Herman James ..5 Neighboring Indian Groups .. 5 Informants ..5 Orthography ..6 Habitat .. 7 Village Sites ..7 Ethnobotany ..10 Ethnozoology. .16 Mammals .16 Birds. 17 Reptiles and batrachians .19 Fishes . 19 Insects and other terrestrial invertebrates. 20 Marine invertebrates .20 Culture Element List .21 Notes on culture element list .38 Appendix: Comparative Notes on Two Historic Village Sites by Clement W. Meighan. 46 Works Cited .............................................. 48 [ v ] ETHNOGRAPHIC NOTES ON THE SOUTHWESTERN POMO BY E. W. GIFFORD INTRODUCTION* The Southwestern Pomo were among the most primitive Charles Haupt married a woman from Chibadono of the California aborigines, a fact to be correlated with [ci'?bad6no] (near Plantation and on the same ridge). She their mountainous terrain on a rugged, inhospitable coast. was called Pashikokoya [pasilk6?koya? ], "cocoon woman" Their low culture may be contrasted with the richer cul- (pashikoyoyu [pa'si-oyo-yu], cocoon used on shaman' s ture of the Pomo of the Russian River Valley and Clear rattle; ya?, personal suffix), but her English name was Lake, environments which offered opportunities for Molly. -

Making Fire with Flint and Steel
Making Fire with Flint and Steel. Early Failures Many survival or scouting books give different instructions on how one can start a fire with flint and steel. These books suggest various materials that are supposed to catch the spark (punk – the powdery dry rot from the insides of fallen logs, cottonwood fluff, fine dry grass, fine wood shavings, dry moss, and various lichens. The people who wrote those books had obviously never tried it. None of these materials work well, although they all make excellent small kindling once you have a fire started. Problem Solved The Oxford Universal Dictionary defines tinder as "a flammable substance used to kindle a fire, especially charred linen". When a spark hits charred cloth, it creates a tiny red spot, which slowly grows like a glowing fairy ring. It is impossible to blow out; in fact, the more wind there is the better, as the spark simply gets hotter and hotter. The only way to put it out is by suffocation (which preserves the rest of the charred cloth for future use), or by dousing it with water (which ruins the char cloth). The amazing thing is that charred cloth in windy weather makes it easier to start a fire with flint and steel than a match. Well, almost! Making Charred Cloth Here is how to put together your own tinder box, so that you can make a fire the same way that people did two hundred years ago. First, you will need some cloth. Linen is the traditional fabric, but 100% cotton works just fine, and it is a lot cheaper! Do not use synthetic or synthetic blend fabrics. -

Low-Impact Living Initiative
firecraft what is it? It's starting and managing fire, which requires fuel, oxygen and ignition. The more natural methods usually progress from a spark to an ember to a flame in fine, dry material (tinder), to small, thin pieces of wood (kindling) and then to firewood. Early humans collected embers from forest fires, lightning strikes and even volcanic activity. Archaeological evidence puts the first use of fire between 200-400,000 years ago – a time that corresponds to a change in human physique consistent with food being cooked - e.g. smaller stomachs and jaws. The first evidence of people starting fires is from around 10,000 years ago. Here are some ways to start a fire. Friction: rubbing things together to create friction Sitting around a fire has been a relaxing, that generates heat and produces embers. An comforting and community-building activity for example is a bow-drill, but any kind of friction will many millennia. work – e.g. a fire-plough, involving a hardwood stick moving in a groove in a piece of softwood. what are the benefits? Percussion: striking things together to make From an environmental perspective, the more sparks – e.g. flint and steel. The sharpness of the natural the method the better. For example, flint creates sparks - tiny shards of hot steel. strikers, fire pistons or lenses don’t need fossil Compression: fire pistons are little cylinders fuels or phosphorus, which require the highly- containing a small amount of tinder, with a piston destructive oil and chemical industries, and that is pushed hard into the cylinder to compress friction methods don’t require the mining, factories the air in it, which raises pressure and and roads required to manufacture anything at all.