HPV Vaccine: Gardasil
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Even Partial COVID-19 Vaccination Protects Nursing Home Residents
News & Analysis News From the Centers for Disease Control and Prevention Even Partial COVID-19 Vaccination lence of underlying medical conditions,” the Protects Nursing Home Residents authors wrote. A CDC analysis has shown that a single dose Waning COVID-19 cases as more resi- of the Pfizer-BioNTech COVID-19 vaccine dents and staff received second vaccina- protected medically vulnerable nursing tions made it impossible to assess vaccine ef- home residents as well as it did general adult fectiveness after 2 doses. Evidence from populations that were evaluated in other ef- previous studies demonstrated greater pro- ficacy and effectiveness studies. tection among older adults after a second The analysis helps fill a data gap about dose, suggesting that completing the 2-dose vaccine effectiveness in this high-risk regimen may be particularly important to group—generally older, frail adults with un- protect long-term care facility residents, the derlying health conditions—who were left authors suggested. out of COVID-19 vaccine trials. Excluding older adults from the trials raised questions Vaccine Dramatically Reduces HPV about how well nursing home residents Infection Among Young Women would respond to vaccination. Widespread vaccination of young women By analyzing the Pfizer-BioNTech vac- against human papillomavirus (HPV) has led cine’sperformanceduringalateJanuaryout- to a greater than 80% reduction in infec- the 4 HPV strains most likely to cause dis- break at 2 Connecticut skilled nursing facili- tions with the 4 strains most often associ- ease. Newer versions that protect against 9 ties, investigators from the CDC and the ated with disease, according to a CDC study. -

HPV Vaccine Safety - Vaccine Safety
CDC - HPV Vaccine Safety - Vaccine Safety Skip directly to search Skip directly to A to Z list Skip directly to navigation Skip directly to site content Skip directly to page options CDC Home CDC 24/7: Saving Lives. Protecting People.™ Search The CDC Vaccine Safety Vaccine Safety Vaccines Safety Basics Diphtheria, Tetanus, and Pertussis Vaccines: DTaP, Td, and Tdap Haemophilus Influenzae Type B (Hib) Hib Summary Human Papillomavirus (HPV) FAQs about HPV Vaccine Safety FAQ: Gardasil Vaccine recall JAMA Report Summary Information from FDA and CDC on Gardasil and its Safety (Archived) Measles, Mumps, Rubella (MMR) MMR Vaccine Safety Studies Measles, Mumps, Rubella, Varicella (MMRV) Information on the VSD MMRV Vaccine Safety Study MMRV Vaccine Safety Studies MMRV and Febrile Seizures Rotavirus Varicella Addressing Common Concerns Adjuvants Autism Vaccine not associated with autism CDC Statement: 2004 Pediatrics Paper CDC Statement on Pandemrix Fainting (Syncope) FAQs about Fainting (Syncope) After Vaccination Childhood Vaccines and Febrile Seizures Influenza Season 2012-2013 Influenza Season 2010-2011 GBS GBS and Menactra Meningococcal Vaccine FAQs about GBS and Menactra Meningococcal Vaccine IOM Assessment of Studies on Childhood Immunization Schedule IOM Report on Adverse Effects of Vaccines Pregnancy and Influenza Vaccine Safety Sudden Infant Death Syndrome (SIDS) Thimerosal http://www.cdc.gov/vaccinesafety/vaccines/HPV/index.html[10/13/2014 5:59:00 PM] CDC - HPV Vaccine Safety - Vaccine Safety CDC Study on Thimerosal and Risk of Autism -

The Hepatitis B Vaccine Is the Most Widely Used Vaccine in the World, with Over 1 Billion Doses Given
Hepatitis B Vaccine Protect Yourself and Those You Love What is Hepatitis B? Hepatitis B is the most common serious liver infection in the world. It is caused by the hepatitis B virus (HBV), which attacks liver cells and can lead to liver failure, cirrhosis (scarring), or liver cancer later in life. The virus is transmitted through direct contact with infected blood and bodily fluids, and from an infected woman to her newborn at birth. Is there a safe vaccine for hepatitis B? YES! The good news is that there is a safe and effective vaccine for hepatitis B. The vaccine is a series, typically given as three shots over a six-month period that will provide a lifetime of protection. You cannot get hepatitis B from the vaccine – there is no human blood or live virus in the vaccine. The hepatitis B vaccine is the most widely used vaccine in the world, with over 1 billion doses given. The hepatitis B vaccine is the first "anti-cancer" vaccine because it can help prevent liver cancer! Who should be vaccinated against hepatitis B? The World Health Organization (WHO) and the U.S. Centers for Disease Control and Prevention (CDC) recommend the hepatitis B vaccine for all newborns and children up to 18 years of age, and all high-risk adults. All infants should receive the first dose of the vaccine in the delivery room or in the first 24 hours of life, preferably within 12 hours. (CDC recommends the first dose within 12 hours vs. the WHO recommendation of 24 hours.) The HBV vaccine is recommended to anyone who is at high risk of infection. -

COVID-19 and Cancer: from Basic Mechanisms to Vaccine Development Using Nanotechnology
International Immunopharmacology 90 (2021) 107247 Contents lists available at ScienceDirect International Immunopharmacology journal homepage: www.elsevier.com/locate/intimp Review COVID-19 and cancer: From basic mechanisms to vaccine development using nanotechnology Hyun Jee Han a,*, Chinekwu Nwagwu b, Obumneme Anyim c, Chinedu Ekweremadu d, San Kim e a University College London, Department of Neonatology, United Kingdom b Department of Pharmaceutics, University of Nigeria Nsukka, Nigeria c Department of Internal Medicine, University of Nigeria Teaching Hospital Ituku-Ozalla, Enugu, Nigeria d Department of Pharmaceutics and Pharmaceutical Technology Enugu State University of Science and Technology, Nigeria e Basildon and Thurrock University Hospital, United Kingdom ARTICLE INFO ABSTRACT Keywords: Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV- COVID-19 2), is a global pandemic which has induced unprecedented ramifications,severely affecting our society due to the Cancer long incubation time, unpredictably high prevalence and lack of effective vaccines. One of the interesting notions Vaccine development is that there is an association between COVID-19 and cancer. Cancer patients seem to exhibit exacerbated Pharmaceutics conditions and a higher mortality rate when exposed to the virus. Therefore, vaccines are the promising solution Nanotechnology to minimise the problem amongst cancer patients threatened by the new viral strains. However, there are still limitations to be considered, including the efficacy of COVID vaccines for immunocompromised individuals, possible interactions between the vaccine and cancer, and personalised medicine. Not only to eradicate the pandemic, but also to make it more effective for immunocompromised patients who are suffering from cancer, a successful vaccine platform is required through the implementation of nanotechnology which can also enable scalable manufacturing and worldwide distribution along with its faster and precise delivery. -

HPV – Facts About the Virus, the Vaccine and What This Means For
The WHO Regional The World Health Organization (WHO) is a specialized agency of the United Nations created in 1948 with the primary responsibility for international health matters each with its own programme geared to the particular health conditions of the countries it serves. Member States Albania Andorra Armenia Austria Azerbaijan Belarus Belgium Bosnia and Herzegovina Bulgaria Croatia Cyprus Czechia HPV – facts about the virus, Denmark Estonia Finland France the vaccine and what this Georgia Germany Greece means for you Hungary Iceland Ireland Israel Italy Kazakhstan Kyrgyzstan Answers to common questions Latvia Lithuania asked by adolescents and Luxembourg Malta young adults Monaco Montenegro Netherlands Norway Poland Portugal Republic of Moldova Romania Russian Federation San Marino Serbia Slovakia Slovenia Spain Sweden Switzerland Tajikistan The former Yugoslav Republic of Macedonia Turkey Turkmenistan Ukraine United Kingdom UN City, Marmorvej 51, DK-2100 Copenhagen Ø, Denmark Uzbekistan Tel.: +45 45 33 70 00 Fax: +45 45 33 70 01 E-mail: [email protected] Q&A: Answers to common questions asked by adolescents and young adults HPV and vaccination HPV stands for human papillomavirus. There are over What is HPV and why should I be vaccinated 200 known types of the virus, 30 of which are transmitted against it? through sexual activity. HPV is the most common sexually transmitted infection in the world. Almost 80% of sexually active people will get infected with one or more HPV types in their lifetime. HPV infection can lead to several types of cancer and genital warts. Cervical cancer is the most common type of cancer caused by HPV, and it is the fourth most common cancer in women worldwide. -

Vaccines for Preteens
| DISEASES and the VACCINES THAT PREVENT THEM | INFORMATION FOR PARENTS Vaccines for Preteens: What Parents Should Know Last updated JANUARY 2017 Why does my child need vaccines now? to get vaccinated. The best time to get the flu vaccine is as soon as it’s available in your community, ideally by October. Vaccines aren’t just for babies. Some of the vaccines that While it’s best to be vaccinated before flu begins causing babies get can wear off as kids get older. And as kids grow up illness in your community, flu vaccination can be beneficial as they may come in contact with different diseases than when long as flu viruses are circulating, even in January or later. they were babies. There are vaccines that can help protect your preteen or teen from these other illnesses. When should my child be vaccinated? What vaccines does my child need? A good time to get these vaccines is during a yearly health Tdap Vaccine checkup. Your preteen or teen can also get these vaccines at This vaccine helps protect against three serious diseases: a physical exam required for sports, school, or camp. It’s a tetanus, diphtheria, and pertussis (whooping cough). good idea to ask the doctor or nurse every year if there are any Preteens should get Tdap at age 11 or 12. If your teen didn’t vaccines that your child may need. get a Tdap shot as a preteen, ask their doctor or nurse about getting the shot now. What else should I know about these vaccines? These vaccines have all been studied very carefully and are Meningococcal Vaccine safe. -

Considerations for Causality Assessment of Neurological And
Occasional essay J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp-2021-326924 on 6 August 2021. Downloaded from Considerations for causality assessment of neurological and neuropsychiatric complications of SARS- CoV-2 vaccines: from cerebral venous sinus thrombosis to functional neurological disorder Matt Butler ,1 Arina Tamborska,2,3 Greta K Wood,2,3 Mark Ellul,4 Rhys H Thomas,5,6 Ian Galea ,7 Sarah Pett,8 Bhagteshwar Singh,3 Tom Solomon,4 Thomas Arthur Pollak,9 Benedict D Michael,2,3 Timothy R Nicholson10 For numbered affiliations see INTRODUCTION More severe potential adverse effects in the open- end of article. The scientific community rapidly responded to label phase of vaccine roll- outs are being collected the COVID-19 pandemic by developing novel through national surveillance systems. In the USA, Correspondence to SARS- CoV-2 vaccines (table 1). As of early June Dr Timothy R Nicholson, King’s roughly 372 adverse events have been reported per College London, London WC2R 2021, an estimated 2 billion doses have been million doses, which is a lower rate than expected 1 2LS, UK; timothy. nicholson@ administered worldwide. Neurological adverse based on the clinical trials.6 kcl. ac. uk events following immunisation (AEFI), such as In the UK, adverse events are reported via the cerebral venous sinus thrombosis and demyelin- MB and AT are joint first Coronavirus Yellow Card reporting website. As of ating episodes, have been reported. In some coun- authors. early June 2021, approximately 250 000 Yellow tries, these have led to the temporary halting of BDM and TRN are joint senior Cards have been submitted, equating to around authors. -

Supplemental Information and Guidance for Vaccination Providers Regarding Use of 9-Valent HPV Vaccine
Supplemental information and guidance for vaccination providers regarding use of 9-valent HPV A 9-valent human papillomavirus (HPV) vaccine (9vHPV, Gardasil 9, Merck & Co.) was licensed for use in females and males in December 2014.1,2,3,4 The 9vHPV was the third HPV vaccine licensed in the United States by the Food and Drug Administration (FDA); the other vaccines are bivalent HPV vaccine (2vHPV, Cervarix, GlaxoSmithKline), licensed for use in females, and quadrivalent HPV vaccine (4vHPV, Gardasil, Merck & Co.), licensed for use in females and males.5 In February 2015, the Advisory Committee on Immunization Practices (ACIP) recommended 9vHPV as one of three HPV vaccines that can be used for routine vaccination of females and one of two HPV vaccines for routine vaccination of males.6 After the end of 2016, only 9vHPV will be distributed in the United States. In October 2016, ACIP updated HPV vaccination recommendations regarding dosing schedules.7 CDC now recommends two doses of HPV vaccine (0, 6–12 month schedule) for persons starting the vaccination series before the 15th birthday. Three doses of HPV vaccine (0, 1–2, 6 month schedule) continue to be recommended for persons starting the vaccination series on or after the 15th birthday and for persons with certain immunocompromising conditions. Guidance is needed for persons who started the series with 2vHPV or 4vHPV and may be completing the series with 9vHPV. The information below summarizes some of the recommendations included in ACIP Policy Notes and provides additional guidance.5-7 Information about the vaccines What are some of the similarities and differences between the three HPV vaccines? y Each of the three HPV vaccines is a noninfectious, virus-like particle (VLP) vaccine. -
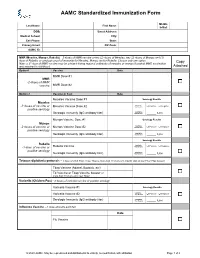
AAMC Standardized Immunization Form
AAMC Standardized Immunization Form Middle Last Name: First Name: Initial: DOB: Street Address: Medical School: City: Cell Phone: State: Primary Email: ZIP Code: AAMC ID: MMR (Measles, Mumps, Rubella) – 2 doses of MMR vaccine or two (2) doses of Measles, two (2) doses of Mumps and (1) dose of Rubella; or serologic proof of immunity for Measles, Mumps and/or Rubella. Choose only one option. Copy Note: a 3rd dose of MMR vaccine may be advised during regional outbreaks of measles or mumps if original MMR vaccination was received in childhood. Attached Option1 Vaccine Date MMR Dose #1 MMR -2 doses of MMR vaccine MMR Dose #2 Option 2 Vaccine or Test Date Measles Vaccine Dose #1 Serology Results Measles Qualitative -2 doses of vaccine or Measles Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Mumps Vaccine Dose #1 Serology Results Mumps Qualitative -2 doses of vaccine or Mumps Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Serology Results Rubella Qualitative Positive Negative -1 dose of vaccine or Rubella Vaccine Titer Results: positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Tetanus-diphtheria-pertussis – 1 dose of adult Tdap; if last Tdap is more than 10 years old, provide date of last Td or Tdap booster Tdap Vaccine (Adacel, Boostrix, etc) Td Vaccine or Tdap Vaccine booster (if more than 10 years since last Tdap) Varicella (Chicken Pox) - 2 doses of varicella vaccine or positive serology Varicella Vaccine #1 Serology Results Qualitative Varicella Vaccine #2 Titer Results: Positive Negative Serologic Immunity (IgG antibody titer) Quantitative Titer Results: _____ IU/ml Influenza Vaccine --1 dose annually each fall Date Flu Vaccine © 2020 AAMC. -

Hepatitis B Vaccine – Frequently Asked Questions (Information from the CDC)
AAMC Standardized Immunization Form 2020 Hepatitis B Vaccine – Frequently Asked Questions (Information from the CDC) 1. What are the hepatitis B vaccines licensed for use in the United States? Three single-antigen vaccines and two combination vaccines are currently licensed in the United States. Single-antigen hepatitis B vaccines: • ENGERIX-B® • RECOMBIVAX HB® • HEPLISAV-B™ Combination vaccines: • PEDIARIX®: Combined hepatitis B, diphtheria, tetanus, acellular pertussis (DTaP), and inactivated poliovirus (IPV) vaccine. Cannot be administered before age 6 weeks or after age 7 years. • TWINRIX®: Combined Hepatitis A and hepatitis B vaccine. Recommended for people aged ≥18 years who are at increased risk for both HAV and HBV infections. 2. What are the recommended schedules for hepatitis B vaccination? The vaccination schedule most often used for children and adults is three doses given at 0, 1, and 6 months. Alternate schedules have been approved for certain vaccines and/or populations. A new formulation, Heplisav-B (HepB-CpG), is approved to be given as two doses one month apart. 3. If there is an interruption between doses of hepatitis B vaccine, does the vaccine series need to be restarted? No. The series does not need to be restarted but the following should be considered: • If the vaccine series was interrupted after the first dose, the second dose should be administered as soon as possible. • The second and third doses should be separated by an interval of at least 8 weeks. • If only the third dose is delayed, it should be administered as soon as possible. 4. Is it harmful to administer an extra dose of hepatitis B vaccine or to repeat the entire vaccine series if documentation of the vaccination history is unavailable or the serology test is negative? No, administering extra doses of single-antigen hepatitis B vaccine is not harmful. -

COVID-19 Vaccines Frequently Asked Questions
Page 1 of 12 COVID-19 Vaccines 2020a Frequently Asked Questions Michigan.gov/Coronavirus The information in this document will change frequently as we learn more about COVID-19 vaccines. There is a lot we are learning as the pandemic and COVID-19 vaccines evolve. The approach in Michigan will adapt as we learn more. September 29, 2021. Quick Links What’s new | Why COVID-19 vaccination is important | Booster and additional doses | What to expect when you get vaccinated | Safety of the vaccine | Vaccine distribution/prioritization | Additional vaccine information | Protecting your privacy | Where can I get more information? What’s new − Pfizer booster doses recommended for some people to boost waning immunity six months after completing the Pfizer vaccine. Why COVID-19 vaccination is important − If you are fully vaccinated, you don’t have to quarantine after being exposed to COVID-19, as long as you don’t have symptoms. This means missing less work, school, sports and other activities. − COVID-19 vaccination is the safest way to build protection. COVID-19 is still a threat, especially to people who are unvaccinated. Some people who get COVID-19 can become severely ill, which could result in hospitalization, and some people have ongoing health problems several weeks or even longer after getting infected. Even people who did not have symptoms when they were infected can have these ongoing health problems. − After you are fully vaccinated for COVID-19, you can resume many activities that you did before the pandemic. CDC recommends that fully vaccinated people wear a mask in public indoor settings if they are in an area of substantial or high transmission. -

Immunogenicity and Safety of a Third Dose, and Immune Persistence Of
medRxiv preprint doi: https://doi.org/10.1101/2021.07.23.21261026; this version posted July 25, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission. 1 Immunogenicity and safety of a third dose, and immune persistence of 2 CoronaVac vaccine in healthy adults aged 18-59 years: interim results 3 from a double-blind, randomized, placebo-controlled phase 2 clinical 4 trial 5 6 Hongxing Pan MSc1*, Qianhui Wu MPH2*, Gang Zeng Ph.D.3*, Juan Yang Ph.D.1, Deyu 7 Jiang MSc4, Xiaowei Deng MSc2, Kai Chu MSc1, Wen Zheng BSc2, Fengcai Zhu M.D.5†, 8 Hongjie Yu M.D. Ph.D.2,6,7†, Weidong Yin MBA8† 9 10 Affiliations 11 1. Vaccine Evaluation Institute, Jiangsu Provincial Center for Disease Control and 12 Prevention, Nanjing, China 13 2. School of Public Health, Fudan University, Key Laboratory of Public Health Safety, 14 Ministry of Education, Shanghai, China 15 3. Clinical Research Department, Sinovac Biotech Co., Ltd., Beijing, China 16 4. Covid-19 Vaccine Department, Sinovac Life Sciences Co., Ltd., Beijing, China 17 5. Jiangsu Provincial Center for Disease Control and Prevention, Nanjing, China 18 6. Shanghai Institute of Infectious Disease and Biosecurity, Fudan University, 19 Shanghai, China 20 7. Department of Infectious Diseases, Huashan Hospital, Fudan University, 21 Shanghai, China 22 8. Sinovac Biotech Co., Ltd., Beijing, China NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice.