Essential Oils and Oil from Seeds of Syagrus Coronata
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effect of Parity on Fatty Acids of Saudi Camels Milk and Colostrum
International Journal of Research in Agricultural Sciences Volume 4, Issue 6, ISSN (Online): 2348 – 3997 Effect of Parity on Fatty Acids of Saudi Camels Milk and Colostrum Magdy Abdelsalam1,2*, Mohamed Ali1 and Khalid Al-Sobayil1 1Department of Animal Production and Breeding, College of Agriculture and Veterinary Medicine, Qassim University, Al-Qassim 51452, Saudi Arabia. 2Department of Animal Production, Faculty of Agriculture, Alexandria University, El-Shatby, Alexandria 21545, Egypt. Date of publication (dd/mm/yyyy): 29/11/2017 Abstract – Fourteen Saudi she-camels were machine milked locations and different feeding regimes, but there is a scare twice daily and fatty acids of colostrum (1-7 days post partum) on the effect of parity of lactating camels on the fatty acids. and milk (10-150 days post partum) were analyzed. Short Therefore, the objective of this experiment was to study the chain fatty acids were found in small percentage in colostrums changes in the fatty acids profile of colostrums and milk of and milk at different parities without insignificant differences she-camel during the first three parities. and the C4:0 and C6:0 don't appear in the analysis. Colostrums has higher unsaturated fatty acids percentage than that of saturated fatty acids while the opposite was found II. MATERIALS AND METHODS in milk of camels. Myiristic acid (C14:0), palmitic (C16:0), stearic (C18:0) and oleic (C18:1) showed the highest A. Animals and Management percentage in either colostrums or milk of she-camels. Parity The present study was carried out on fourteen Saudi she had significant effect on atherogenicity index (AI) which is camels raised at the experimental Farm, College of considered an important factor associated the healthy quality of camel milk. -

Retention Indices for Frequently Reported Compounds of Plant Essential Oils
Retention Indices for Frequently Reported Compounds of Plant Essential Oils V. I. Babushok,a) P. J. Linstrom, and I. G. Zenkevichb) National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA (Received 1 August 2011; accepted 27 September 2011; published online 29 November 2011) Gas chromatographic retention indices were evaluated for 505 frequently reported plant essential oil components using a large retention index database. Retention data are presented for three types of commonly used stationary phases: dimethyl silicone (nonpolar), dimethyl sili- cone with 5% phenyl groups (slightly polar), and polyethylene glycol (polar) stationary phases. The evaluations are based on the treatment of multiple measurements with the number of data records ranging from about 5 to 800 per compound. Data analysis was limited to temperature programmed conditions. The data reported include the average and median values of retention index with standard deviations and confidence intervals. VC 2011 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. [doi:10.1063/1.3653552] Key words: essential oils; gas chromatography; Kova´ts indices; linear indices; retention indices; identification; flavor; olfaction. CONTENTS 1. Introduction The practical applications of plant essential oils are very 1. Introduction................................ 1 diverse. They are used for the production of food, drugs, per- fumes, aromatherapy, and many other applications.1–4 The 2. Retention Indices ........................... 2 need for identification of essential oil components ranges 3. Retention Data Presentation and Discussion . 2 from product quality control to basic research. The identifi- 4. Summary.................................. 45 cation of unknown compounds remains a complex problem, in spite of great progress made in analytical techniques over 5. -

WO 2017/074902 Al 4 May 20 17 (04.05.2017) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2017/074902 Al 4 May 20 17 (04.05.2017) W P O P C T (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 8/37 (2006.01) A61Q 19/00 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, A61K 31/215 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (21) International Application Number: KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, PCT/US2016/058591 MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (22) International Filing Date: OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, 25 October 2016 (25.10.201 6) SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (25) Filing Language: English ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 62/247,803 29 October 20 15 (29. 10.20 15) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: GLAXOSMITHKLINE CONSUMER TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, HEALTHCARE HOLDINGS (US) LLC [US/US]; 271 1 DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, Centerville Road, Suite 400, Wilmington, DE 19808 (US). -
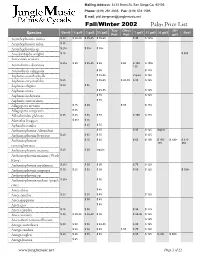
Winter-Fall Sale 2002 Palm Trees-Web
Mailing Address: 3233 Brant St. San Diego Ca, 92103 Phone: (619) 291 4605 Fax: (619) 574 1595 E mail: [email protected] Fall/Winter 2002 Palm Price List Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Acanthophoenix crinita $ 30 $ 30-40 $ 35-45 $ 55-65 $ 95 $ 125+ Acanthophoenix rubra $ 35 Acanthophoenix sp. $ 25+ $ 35+ $ 55+ Acoelorrhaphe wrightii $ 15 $ 300 Acrocomia aculeata $ 25+ $ 35 $ 35-45 $ 65 $ 65 $ 100- $ 150+ Actinokentia divaricata 135 Actinorhytis calapparia $ 55 $ 125 Aiphanes acanthophylla $ 45-55 inquire $ 125 Aiphanes caryotaefolia $ 25 $ 55-65 $ 45-55 $ 85 $ 125 Aiphanes elegans $ 20 $ 35 Aiphanes erosa $ 45-55 $ 125 Aiphanes lindeniana $ 55 $ 125 Aiphanes vincentsiana $ 55 Allagoptera arenaria $ 25 $ 40 $ 55 $ 135 Allagoptera campestris $ 35 Alloschmidtia glabrata $ 35 $ 45 $ 55 $ 85 $ 150 $ 175 Alsmithia longipes $ 35+ $ 55 Aphandra natalia $ 35 $ 55 Archontophoenix Alexandrae $ 55 $ 85 $ 125 inquire Archontophoenix Beatricae $ 20 $ 35 $ 55 $ 125 Archontophoenix $ 25 $ 45 $ 65 $ 100 $ 150- $ 200+ $ 310- 175 350 cunninghamiana Archontophoenix maxima $ 25 $ 30 inquire Archontophoenix maxima (Wash River) Archontophoenix myolaensis $ 25+ $ 30 $ 50 $ 75 $ 125 Archontophoenix purpurea $ 30 $ 25 $ 35 $ 50 $ 85 $ 125 $ 300+ Archontophoenix sp. Archontophoenix tuckerii (peach $ 25+ $ 55 river) Areca alicae $ 45 Areca catechu $ 20 $ 35 $ 45 $ 125 Areca guppyana $ 30 $ 45 Areca ipot $ 45 Areca triandra $ 25 $ 30 $ 95 $ 125 Areca vestiaria $ 25 $ 30-35 $ 35-40 $ 55 $ 85-95 $ 125 Arecastrum romanzoffianum $ 125 Arenga australasica $ 20 $ 30 $ 35 $ 45-55 $ 85 $ 125 Arenga caudata $ 20 $ 30 $ 45 $ 55 $ 75 $ 100 Arenga engleri $ 20 $ 60 $ 35 $ 45 $ 85 $ 125 $ 200 $ 300+ Arenga hastata $ 25 www.junglemusic.net Page 1 of 22 Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Arenga hookeriana inquire Arenga micranthe 'Lhutan' $ 20 inquire Arenga pinnata $ 35 $ 50 $ 85 $ 125 Arenga sp. -

Improvement of Lipid Production from an Oil-Producing Filamentous Fungus, Penicillium Brevicompactum NRC 829, Through Central Composite Statistical Design
Ann Microbiol (2017) 67:601–613 DOI 10.1007/s13213-017-1287-x ORIGINAL ARTICLE Improvement of lipid production from an oil-producing filamentous fungus, Penicillium brevicompactum NRC 829, through central composite statistical design Thanaa H. Ali1 & Mamdouh S. El-Gamal2 & Dina H. El-Ghonemy1 & Ghada E. Awad3 & Amir E. Tantawy1 Received: 12 March 2017 /Accepted: 13 July 2017 /Published online: 7 August 2017 # Springer-Verlag GmbH Germany and the University of Milan 2017 Abstract In the present study, 13 filamentous fungi were commercial development for the production of LA by fer- screened for their lipid production and an oleaginous fun- mentation using cheap raw material. gus, Penicillium brevicompactum NRC 829, was found to be the highest lipid producer. Screening of various agro- Keywords Linoleic acid . Penicillium brevicompactum NRC industrial residues was performed and sunflower oil cake 829 . Response surface methodology . Unsaturated fatty acids proved to be the best substrate for lipid production. A central composite design was employed to investigate the optimum concentrations of the most significant medi- Introduction um components required to improve the lipid production by P. brevicompactum. The results clearly revealed that Polyunsaturated fatty acids (PUFAs) are long-chain fatty − the maximal lipid production of 8.014 ± 0.06 gL 1 acids containing two or more double bonds in their acyl (representing 57.6% lipid/dry biomass) was achieved by chains. Biosynthesis of PUFAs involves both methyl- the fungus when grown for 6 days at 30 °C under static directed and carboxyl-directed desaturases. The primary condition in a medium containing sunflower oil cake, product of fatty acid biosynthesis in oilseed crops is the NaNO3 and KCl at final concentrations of 8, 0.75 and 18-carbon monounsaturated oleic acid (C18:1–9). -

National Food Safety Standard Determination of Fatty Acids in Foods
National Standard of the People’s Republic of China GB 5413.27 – 2010 National food safety standard Determination of fatty acids in foods for infants and young children, milk and milk products Issued on: 2010-03-26 Implemented on: 2010-06-01 Issued by the Ministry of Health of People’s Republic of China GB 5413.27–2010 Preface This standard replaces GB/T 21676 - 2008 Determination of Fatty Acids in Formula Foods and Milk Powder for Infants and Young Children, GB/T 5413.27 – 1997 Determination of DHA and EPA in Formula Foods and Milk Powder for Infants and Young Children and GB/T 5413.4 - 1997 Determination of Linoleic Acid in Formula Foods and Milk Powder for Infants and Young Children. Comparing with the original standards, the following main changes have been made to the Standard: the first method is Acetyl Chloride - Methanol Esterification; Integrate GB/T 21676 - 2008, GB/T 5413.27 – 1997 and GB/T 5413.4 – 1997 to the second method of this standard, Ammonia Water - Ethanol Extraction Method. Appendix A of this standard is informative. The versions replaced by this standard are: - GB/T 5413.4 – 1997, GB/T 5413.27 - 1997; - GB/T 21676 - 2008. 1 GB 5413.27–2010 National food safety standard Determination of fatty acids in foods for infants and young children, milk and milk products 1 Scope This standard provides the determination of fatty acids in infant foods and dairy. This standard applies to determination of fatty acids in infant foods and dairy; the second method doesn’t apply to determination of embedded fatty acid. -

Biochemistry Prologue to Lipids
Paper : 05 Metabolism of Lipids Module: 01 Prologue to Lipids Principal Investigator Dr. Sunil Kumar Khare, Professor, Department of Chemistry, IIT-Delhi Paper Coordinator and Dr. Suaib Luqman, Scientist (CSIR-CIMAP) Content Writer & Assistant Professor (AcSIR) CSIRDr. Vijaya-CIMAP, Khader Lucknow Dr. MC Varadaraj Content Reviewer Prof. Prashant Mishra, Professor, Department of Biochemical Engineering and Biotechnology, IIT-Delhi 1 METABOLISM OF LIPIDS Biochemistry Prologue to Lipids DESCRIPTION OF MODULE Subject Name Biochemistry Paper Name 05 Metabolism of Lipids Module Name/Title 01 Prologue to Lipids 2 METABOLISM OF LIPIDS Biochemistry Prologue to Lipids 1. Objectives To understand what is lipid Why they are important How they occur in nature 2. Concept Map LIPIDS Fatty Acids Glycerol 3. Description 3.1 Prologue to Lipids In 1943, the term lipid was first used by BLOOR, a German biochemist. Lipids are heterogeneous group of compounds present in plants and animal tissues related either actually or potentially to the fatty acids. They are amphipathic molecules, hydrophobic in nature originated utterly or in part by thioesters (carbanion-based condensations of fatty acids and/or polyketides etc) or by isoprene units (carbocation-based condensations of prenols, sterols, etc). Lipids have the universal property of being: i. Quite insoluble in water (polar solvent) ii. Soluble in benzene, chloroform, ether (non-polar solvent) 3 METABOLISM OF LIPIDS Biochemistry Prologue to Lipids Thus, lipids include oils, fats, waxes, steroids, vitamins (A, D, E and K) and related compounds, such as phospholipids, triglycerides, diglycerides, monoglycerides and others, which are allied more by their physical properties than by their chemical assests. -

MORPHOLOGY of FRUITS, DIASPORES, SEEDS, SEEDLINGS, and SAPLINGS of Syagrus Coronata (Mart.) Becc
652 Original Article MORPHOLOGY OF FRUITS, DIASPORES, SEEDS, SEEDLINGS, AND SAPLINGS OF Syagrus coronata (Mart.) Becc. MORFOLOGIA DE FRUTOS, DIÁSPOROS, SEMENTES, PLÂNTULAS E MUDAS DE Syagrus coronata (Mart.) Becc Sueli da Silva SANTOS-MOURA 1; Edilma Pereira GONÇALVES 2; Luan Danilo Ferreira de Andrade MELO 1; Larissa Guimarães PAIVA 1; Tatiana Maria da SILVA 1 1. Master's in Agricultural Production by the Rural Federal University of Pernambuco, Academic Unit of Garanhuns, Garanhuns, PE, Brazil; 2. Teacher, doctor at the Federal Rural University of Pernambuco, Academic Unit of Garanhuns, Garanhuns, PE, Brazil. ABSTRACT: Licuri ( Syagrus coronata (Mart.) Becc.) is an ornamental palm tree native of Brazil with great economic potential, because it provides raw material for manufacturing a wide range of products. The objective of this study was to assess the morphology of the fruits, diaspores, seeds, seedlings, and saplings of Syagrus coronata . The study was performed at the Laboratory of Seed Analysis (LSA) of the Federal Rural University of Pernambuco/Academic Unit of Garanhuns-PE, by using licuri fruits collected from the rural area of Caetés-PE. It was evaluated fruit morphology, diaspores, seeds, seedlings and saplings. Germination, in the form of cotyledon petiole emergence, began 15 days after sowing, is hypogeal, cryptocotylar, and remote tubular. It is slow and uneven, extending up to 60 days after the first eophyll appears. The saplings have alternate, pinnate, glabrous, entire leaves with parallel venation and sheath invagination. The primary roots persistent, the secondary roots arise from the stem root node in the primary root, and lateral roots only fasciculate was evidenced when the change was 300 days, and must remain in the nursery for at least 360 days after germination before taking it to the field, due to the slow development of this species. -

Sfps Fall 2011 Sale Plant List
SFPS FALL 2011 SALE PLANT LIST PLANTS VENDOR # Palms Acanthophoenix rubra 35 Acoelorrhaphe wrightii 26, 67 Acrocomia aculeata 50, 67 Actinokentia divaricata 35, 57, 66, 68, 72 Actinorhytis calapparia 72 Adonidia merrillii 31, 57, 66, 89 Adonidia merrillii var. "Golden Form" 35 Aiphanes aculeata = Aiphanes horrida - Aiphanes caryotifolia = Aiphanes horrida - Aiphanes erosa = Aiphanes minima - Aiphanes horrida 35, 68, 72 Aiphanes minima 68 Aiphanes vincentiana = Aiphanes minima - Allagoptera arenaria 57, 66, 67, 68, 72 Allagoptera campestris 67 Allagoptera leucocalyx 57 Alloschmidia glabrata = Basselinia glabrata - Alsmithia longipes = Heterospathe longipes - Archontophoenix cunninghamiana var. 'Illawara' 68 Archontophoenix maxima 67, 72 Archontophoenix myolensis 50, 66, 67, 68 Archontophoenix purpurea 57, 66, 72 Archontophoenix tuckeri 66, 68 Areca aliceae = Areca triandra - Areca camarinensis 57, 68 Areca catechu 57, 67, 72 Areca catechu var. 'Dwarf' 35, 50 Areca hutchinsoniana 68 Areca ipot 67 Areca latiloba = Areca montana - Areca macrocalyx var. 'Red Form' 35, 57, 68 Areca macrocarpa 68 Areca montana 57 Areca triandra 68, 72 Areca vestiaria 25, 35, 57, 67, 68 Areca vestiaria var. 'Orange Form' 25, 57, 67, 72 Areca vestiaria var. 'Maroon Leaf' 35, 57, 67 Areca vestiaria var. 'Red Leaf' 57, 67, 72 Areca sp. 'Yellow Crownshaft' 25 Arenga ambong = Arenga undulatifolia - Arenga brevipes 57 Arenga caudata 66 Arenga engleri 31, 66, 68, 72 Arenga hookeriana 35, 57, 66, 72 Arenga microcarpa 26, 66 Arenga obtusifolia 57, 66 PLANTS VENDOR # Arenga pinnata 50, 57, 66, 67, 68 Arenga porphyrocarpa 66 Arenga tremula 26, 57, 66, 68, 72 Arenga undulatifolia 35, 57, 66, 67 Arenga westerhoutii 68 Asterogyne martiana 57, 68, 72 Astrocaryum acaule 72 Astrocaryum alatum 35, 50, 57, 67 Astrocaryum mexicanum 72 Astrocaryum murumuru 72 Attalea butyracea 57, 67, 72 Attalea cohune 35 Attalea phalerata 50, 91 Attalea rostrata 68 Attalea speciosa 50, 66 Bactris bidentula 72 Bactris gasipaes 67 Bactris gasipaes var. -

Fast and Simple Free Fatty Acids Analysis Using UPC2/MS Giorgis Isaac,1 Michael D
Fast and Simple Free Fatty Acids Analysis Using UPC2/MS Giorgis Isaac,1 Michael D. Jones,1 Besnik Bajrami,1 Wassim Obeid,2 James Langridge,3 Patrick Hatcher2 1Waters Corporation, Milford, MA, USA 2Old Dominion University, Norfolk, VA, USA 3Waters Corporation, Manchester, UK APPLICATION BENEFITS INTRODUCTION ■■ Demonstrates the separation of free fatty Fatty acids, both free and as part of complex lipids, play a number of key roles acid (FFA) species based on chain length in metabolism – as major metabolic fuel (storage and transport of energy), as and number of double bonds essential components of all membranes, and as gene regulators. In addition, dietary lipids provide polyunsaturated fatty acids that are precursors of powerful ■■ No derivatization is required, which results in easier and fast sample preparation and locally acting metabolites, e.g., eicosanoids. eliminates artifact formation The common fatty acids of animal and plant origin have even-numbered chains ■■ Organic phase lipid extract can be directly of 16 to 24 carbon atoms with 0 to 6 double bonds. Nature provides countless injected onto the system, saving time and exceptions, however, and odd- and even-numbered fatty acids with up to nearly reducing cost per analysis 100 carbon atoms exist. In addition, double bonds can be of the cis (Z) and trans (E) configuration and there can be innumerable other structural features, ■■ Less than three-minute chromatographic including branch points, rings, oxygenated functions, and many more. separation is up to 10X faster compared to GC/MS Fatty acid chains may contain one or more double bonds at specific positions (unsaturated and poly unsaturated with cis (Z) or trans (E) configuration) or they ■■ Unlike GC/MS, low volatile and very long chain fatty acids (>24 carbon atoms) can be may be fully saturated. -

Record of Tritrophic Relationship Between Syagrus Coronata (Martius)
doi: 10.12741/ebrasilis.v14.e922 e-ISSN 1983-0572 Creative Commons License v4.0 (CC-BY) Copyright © Author(s) Article Full Open Access Scientific Note Record of tritrophic relationship between Syagrus coronata (Martius) Beccari (Arecaceae), Pachymerus nucleorum Fabricius (Coleoptera: Chrysomelidae: Bruchinae) and Heterospilus sp. (Hymenoptera: Braconidae) in the State of Alagoas, northeastern Brazil Jefferson Duarte de Melo , Suianne Oliveira dos Santos Cajé & Iracilda Maria de Moura Lima Laboratório de Bioecologia de Insetos, Instituto de Ciências Biológicas e da Saúde, Universidade Federal de Alagoas, Maceió, Alagoas, Brazil. EntomoBrasilis 14: e922 (2021) Edited by: Abstract. Some conservation units in Brazil border urban areas, like the Catolé and Fernão Velho William Costa Rodrigues Environmental Protection Area (EPA) in the State of Alagoas. In urban areas, there is the habit of cultivating plants for landscape purposes, and Syagrus coronata (Martius) Beccari (Arecaceae), “Licuri” Article History: or “Ouricuri”, is a palm tree commonly used in ornamentation; a native species from Caatinga and Received: 23.vii.2020 Atlantic Forest biomes widely explored through time. Some insects have part of their development Accepted: 30.xii.2020 associated with plants, and Pachymerus nucleorum Fabricius (Coleoptera: Chrysomelidae: Bruchinae) Published: 06.iii.2021 has a close connection with some Arecaceae. Females usually lay eggs on the surface of fallen fruits and the immatures feed on the seed under the drupe endocarp; the larvae, even protected by the Corresponding author: hard surface could be preyed by skilled parasitoid wasps. Here, the record of a tritrophic relationship Jefferson Duarte de Melo between S. coronata, P. nucleorum, and a wasp of the genus Heterospilus (Hymenoptera: Braconidae) [email protected] in an urbanized region of Alagoas, close to a remnant of Atlantic Forest of the Catolé and Fernão Funding agencies: Velho EPA is communicated. -

Mass Spectrometry of Astrobiologically Relevant Organic Material – Implications on Future Space Missions to Ocean Worlds in the Outer Solar System
Mass Spectrometry of Astrobiologically Relevant Organic Material – Implications on Future Space Missions to Ocean Worlds in the Outer Solar System Fabian Klenner1, Frank Postberg1, Ferdinand Stolz2, René Reviol1, and Nozair Khawaja1 1Institute of Earth Sciences, Heidelberg University, Germany 2Wilhelm-Ostwald-Institute, Leipzig University, Germany 51st ESLAB Symposium - Extreme Habitable Worlds ESA ESTEC, The Netherlands The Solar System‘s ocean worlds Steve Vance; NASA/JPL-Caltech December 6, 2017 2 The Solar System‘s ocean worlds Steve Vance; NASA/JPL-Caltech December 6, 2017 3 Sampling dust and ice particles in space E ring NASA/JPL December 6, 2017 4 The Cosmic Dust Analyzer (CDA) Chemical Analyzer subsystem (CA) § Sensitive to cations § Determines impact rates, mass, speed, electric charge, and composition § Mass resolution: ≤ 50 m/Dm § Maximum recorded mass: ca. 200 u Srama et al. 2004 December 6, 2017 5 Analog experiment: IR-FL-MALDI-ToF-MS IR Laser adapted from Beinsen 2011 § Liquid beam: r = 7.5 µm § Laser: l = 2480 nm; I ≤ 1152 MW/cm2 § Vaccum chamber: P = 5 x 10-5 mbar § ToF-MS: R = 700 – 800 m/Dm December 6, 2017 6 Comparison of the impact mechanisms of CDA and the analog experiment CDA Analog experiment Postberg et al. 2009 December 6, 2017 7 Comparison of the impact mechanisms of CDA and the analog experiment CDA Analog experiment Postberg et al. 2009 Khawaja 2016 December 6, 2017 8 Future missions and Enceladus Ice Analyzer (ENIA) § Sensitive to cations and anions § Mass resolution > 2000 m/Dm § Maximum recorded mass: 2000