Habitat and Distribution of Post-Recruit Life Stages of the Squid Loligo Forbesii
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Quantifying the Swimming Gaits of Veined Squid (Loligo Forbesii) Using Bio-Logging Tags Genevieve E
© 2019. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2019) 222, jeb198226. doi:10.1242/jeb.198226 RESEARCH ARTICLE Quantifying the swimming gaits of veined squid (Loligo forbesii) using bio-logging tags Genevieve E. Flaspohler1,2, Francesco Caruso3,4, T. Aran Mooney3,*, Kakani Katija5, Jorge Fontes6,7,8, Pedro Afonso6,7,8 and K. Alex Shorter9 ABSTRACT INTRODUCTION Squid are mobile, diverse, ecologically important marine organisms Squid are diverse and ecologically important marine organisms. As whose behavior and habitat use can have substantial impacts on squid are ectothermic animals, their physiology and behavior can be ecosystems and fisheries. However, as a consequence in part of the directly linked to the physical conditions of their surrounding inherent challenges of monitoring squid in their natural marine environment (Kaplan et al., 2013; Rosa and Seibel, 2010). In turn, environment, fine-scale behavioral observations of these free- squid behavior and habitat use can have substantial impacts on swimming, soft-bodied animals are rare. Bio-logging tags provide an marine ecosystems and fisheries. Monitoring and observing squid emerging way to remotely study squid behavior in their natural behavioral patterns and vital rates can be important for ’ environments. Here, we applied a novel, high-resolution bio-logging understanding their activity and energy usage (O Dor et al., 1995; tag (ITAG) to seven veined squid, Loligo forbesii, in a controlled Pörtner, 2002), as well as for elucidating the broader ecological experimental environment to quantify their short-term (24 h) behavioral interactions between squid and the taxa they influence (e.g. foraging patterns. Tag accelerometer, magnetometer and pressure data were rates; Clarke, 1977) and how environmental changes may alter these used to develop automated gait classification algorithms based on activities (Pörtner et al., 2004; Rosa et al., 2014). -

Interim Report of the Working Group on Cephalopod Fisheries and Life History (WGCEPH)
ICES WGCEPH REPORT 2015 SCICOM STEERING GROUP ON ECOSYSTEM PROCESSES AND DYNAMICS ICES CM 2015/SSGEPD:02 REF. SCICOM Interim Report of the Working Group on Cephalopod Fisheries and Life History (WGCEPH) 8-11 June 2015 Tenerife, Spain International Council for the Exploration of the Sea Conseil International pour l’Exploration de la Mer H. C. Andersens Boulevard 44–46 DK-1553 Copenhagen V Denmark Telephone (+45) 33 38 67 00 Telefax (+45) 33 93 42 15 www.ices.dk [email protected] Recommended format for purposes of citation: ICES. 2016. Interim Report of the Working Group on Cephalopod Fisheries and Life History (WGCEPH), 8–11 June 2015, Tenerife, Spain. ICES CM 2015/SSGEPD:02. 127 pp. For permission to reproduce material from this publication, please apply to the Gen- eral Secretary. The document is a report of an Expert Group under the auspices of the International Council for the Exploration of the Sea and does not necessarily represent the views of the Council. © 2016 International Council for the Exploration of the Sea ICES WGCEPH REPORT 2015 | i Contents Executive summary ................................................................................................................ 3 1 Administrative details .................................................................................................. 4 2 Terms of Reference (summarised) and Work plan .................................................. 4 3 List of Outcomes and Achievements of the WG in this delivery period ............ 6 4 Progress report on ToRs and workplan .................................................................... -

What's On? What's Out?
CCIIAACC NNeewwsslleetttteerr Issue 2, September 2010 would like to thank everyone the cephalopod community. So EEddiittoorriiaall Ifor their contributions to this if you find yourself appearing Louise Allcock newsletter. To those who there, don't take it as a slur on responded rapidly back in June your age - but as a compliment to my request for copy I must to your contribution!! apologise. A few articles didn't One idea that I haven't had a make the deadline of 'before my chance to action is a suggestion summer holiday'... Other from Eric Hochberg that we deadlines then had to take compile a list of cephalopod precedence. PhD and Masters theses. I'll Thanks to Clyde Roper for attempt to start this from next suggesting a new section on year. If you have further 'Old Faces' to complement the suggestions, please let me have 'New Faces' section and to them and I'll do my best to Sigurd von Boletzky for writing incorporate them. the first 'Old Faces' piece on Pio And finally, the change in Fioroni. You don't have to be colour scheme was prompted dead to appear in 'Old Faces': in by the death of my laptop and fact you don't actually have to all the Newsletter templates be old - but you do have to have that I had so lovingly created. contributed years of service to Back up? What back up... WWhhaatt''ssoonn?? 9tth - 15tth October 2010 5th International Symposium on Pacific Squid La Paz, BCS, Mexico. 12tth - 17tth June 2011 8th CLAMA (Latin American Congress of Malacology) Puerto Madryn, Argentina See Page 13 for more details 18tth - 22nd June 2011 6th European Malacology Congress Vitoria, Spain 2012 CIAC 2012 Brazil WWhhaatt''ssoouutt?? Two special volumes of cephalopod papers are in nearing completion. -

Environmental Effects on Cephalopod Population Dynamics: Implications for Management of Fisheries
Advances in Cephalopod Science:Biology, Ecology, Cultivation and Fisheries,Vol 67 (2014) Provided for non-commercial research and educational use only. Not for reproduction, distribution or commercial use. This chapter was originally published in the book Advances in Marine Biology, Vol. 67 published by Elsevier, and the attached copy is provided by Elsevier for the author's benefit and for the benefit of the author's institution, for non-commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues who know you, and providing a copy to your institution’s administrator. All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution’s website or repository, are prohibited. For exceptions, permission may be sought for such use through Elsevier's permissions site at: http://www.elsevier.com/locate/permissionusematerial From: Paul G.K. Rodhouse, Graham J. Pierce, Owen C. Nichols, Warwick H.H. Sauer, Alexander I. Arkhipkin, Vladimir V. Laptikhovsky, Marek R. Lipiński, Jorge E. Ramos, Michaël Gras, Hideaki Kidokoro, Kazuhiro Sadayasu, João Pereira, Evgenia Lefkaditou, Cristina Pita, Maria Gasalla, Manuel Haimovici, Mitsuo Sakai and Nicola Downey. Environmental Effects on Cephalopod Population Dynamics: Implications for Management of Fisheries. In Erica A.G. Vidal, editor: Advances in Marine Biology, Vol. 67, Oxford: United Kingdom, 2014, pp. 99-233. ISBN: 978-0-12-800287-2 © Copyright 2014 Elsevier Ltd. Academic Press Advances in CephalopodAuthor's Science:Biology, personal Ecology, copy Cultivation and Fisheries,Vol 67 (2014) CHAPTER TWO Environmental Effects on Cephalopod Population Dynamics: Implications for Management of Fisheries Paul G.K. -
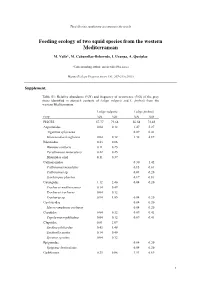
Marine Ecology Progress Series 531:207
The following supplement accompanies the article Feeding ecology of two squid species from the western Mediterranean M. Valls*, M. Cabanellas-Reboredo, I. Uranga, A. Quetglas *Corresponding author: [email protected] Marine Ecology Progress Series 531: 207–219 (2015) Supplement. Table S1: Relative abundance (%N) and frequency of occurrence (%O) of the prey items identified in stomach contents of Loligo vulgaris and L. forbesii from the western Mediterranean. Loligo vulgaris Loligo forbesii Prey %N %O %N %O PISCES 57.77 79.68 56.54 73.43 Argentinidae 0.04 0.12 1.47 5.27 Argentina sphyraena 0.09 0.41 Glossanodon leioglossus 0.04 0.12 1.38 4.87 Blenniidae 0.43 0.86 Blennius ocellaris 0.11 0.25 Parablennius tentacularis 0.22 0.25 Blenniidae unid. 0.11 0.37 Callionymidae 0.30 1.42 Callionymus maculatus 0.13 0.61 Callionymus sp. 0.04 0.20 Synchiropus phaeton 0.17 0.81 Carangidae 1.12 2.46 0.04 0.20 Trachurus mediterraneus 0.14 0.49 Trachurus trachurus 0.04 0.12 Trachurus sp. 0.94 1.85 0.04 0.20 Centriscidae 0.04 0.20 Macroramphosus scolopax 0.04 0.20 Cepolidae 0.04 0.12 0.09 0.41 Cepola macrophthalma 0.04 0.12 0.09 0.41 Clupeidae 0.61 2.09 Sardina pilchardus 0.43 1.48 Sardinella aurita 0.14 0.49 Sprattus sprattus 0.04 0.12 Epigonidae 0.04 0.20 Epigonus denticulatus 0.04 0.20 Gadiformes 0.25 0.86 1.51 6.69 1 Loligo vulgaris Loligo forbesii Prey %N %O %N %O Micromessistius poutassou 0.43 2.03 Molva dypterygia 0.04 0.20 Phycis blennoides 0.22 1.01 Gadiculus argenteus 0.04 0.12 0.56 2.64 Gaidropsarus biscayensis 0.22 0.74 0.26 1.22 Gobiidae 26.81 18.60 32.10 16.02 Aphia minuta 0.58 0.74 26.52 9.74 Pseudoaphya ferreri 0.18 0.25 Crystallogobius linearis 1.84 1.35 3.89 2.23 Deltentosteus quadrimaculatus 0.04 0.20 Gobius niger 0.14 0.37 Lesueurigobius friesii 1.30 1.85 Lesueurigobius sanzi 0.18 0.49 Lesueurigobius suerii 0.04 0.12 Lesueurigobius sp. -

Bio-Ecological Features Update on Eleven Rare Cartilaginous Fish in the Central-Western Mediterranean Sea As a Contribution for Their Conservation
life Article Bio-Ecological Features Update on Eleven Rare Cartilaginous Fish in the Central-Western Mediterranean Sea as a Contribution for Their Conservation Antonello Mulas 1,2,*,†, Andrea Bellodi 1,2,† , Pierluigi Carbonara 3 , Alessandro Cau 1,2 , Martina Francesca Marongiu 1,2 , Paola Pesci 1,2, Cristina Porcu 1,2 and Maria Cristina Follesa 1,2 1 Department of Life and Environmental Sciences, University of Cagliari, 09126 Cagliari, Italy; [email protected] (A.B.); [email protected] (A.C.); [email protected] (M.F.M.); [email protected] (P.P.); [email protected] (C.P.); [email protected] (M.C.F.) 2 CoNISMa Consorzio Nazionale Interuniversitario per le Scienze del Mare, 00196 Roma, Italy 3 COISPA Tecnologia & Ricerca, 70126 Bari, Italy; [email protected] * Correspondence: [email protected] † Authors contributed equally to this work and should be considered co-first authors. Abstract: Cartilaginous fish are commonly recognized as key species in marine ecosystems for their fundamental ecological role as top predators. Nevertheless, effective management plans for cartilagi- nous fish are still missing, due to the lack of knowledge on their abundance, distribution or even life-history. In this regard, this paper aims at providing new information on the life-history traits, such as age, maturity, reproductive period, in addition to diet characteristics of eleven rare cartilagi- Citation: Mulas, A.; Bellodi, A.; nous fish inhabiting the Central-Western Mediterranean Sea belonging to the orders Chimaeriformes Carbonara, P.; Cau, A.; (Chimaera monstrosa), Hexanchiformes (Heptranchias perlo and Hexanchus griseus), Myliobatiformes Marongiu, M.F.; Pesci, P.; Porcu, C.; Follesa, M.C. Bio-Ecological Features (Aetomylaeus bovinus and Myliobatis aquila), Rajiformes (Dipturus nidarosiensis and Leucoraja circu- Update on Eleven Rare Cartilaginous laris), Squaliformes (Centrophorus uyato, Dalatias licha and Oxynotus centrina) and Torpediniformes Fish in the Central-Western (Tetronarce nobiliana), useful for their assessment and for future management actions. -

A Rapid Colorimetric Assay for On-Site Authentication of Cephalopod Species
biosensors Communication A Rapid Colorimetric Assay for On-Site Authentication of Cephalopod Species 1, 1, 2,3 4, Giuseppina Tatulli y, Paola Cecere y, Davide Maggioni , Andrea Galimberti * and Pier Paolo Pompa 1,* 1 Istituto Italiano di Tecnologia, Nanobiointeractions&Nanodiagnostics, Via Morego 30, 16163 Genova, Italy; [email protected] (G.T.); [email protected] (P.C.) 2 Department of Earth and Environmental Sciences (DISAT), University of Milano-Bicocca, P.za Della Scienza 1, 20126 Milan, Italy; [email protected] 3 Marine Research and High Education (MaRHE) Center, University of Milano-Bicocca, Faafu Magoodhoo 12030, Maldives 4 ZooPlantLab, Department of Biotechnology and Biosciences, University of Milano-Bicocca, P.za Della Scienza 2, 20126 Milan, Italy * Correspondence: [email protected] (A.G.); [email protected] (P.P.P.) These authors contributed equally to this work. y Received: 19 October 2020; Accepted: 19 November 2020; Published: 24 November 2020 Abstract: A colorimetric assay, exploiting the combination of loop-mediated isothermal amplification (LAMP) with DNA barcoding, was developed to address the authentication of some cephalopod species, a relevant group in the context of seafood traceability, due to the intensive processing from the fishing sites to the shelf. The discriminating strategy relies on accurate design of species-specific LAMP primers within the conventional 5’ end of the mitochondrial COI DNA barcode region and allows for the identification of Loligo vulgaris among two closely related and less valuable species. The assay, coupled to rapid genomic DNA extraction, is suitable for large-scale screenings and on-site applications due to its easy procedures, with fast (30 min) and visual readout. -

Cephalopod Biology and Fisheries in Europe: II. Species Accounts | 115
114 | ICES Cooperative Research Report No. 325 Cephalopod biology and fisheries in European waters: species accounts Loligo vulgaris European squid Cephalopod biology and fisheries in Europe: II. Species Accounts | 115 11 Loligo vulgaris Lamarck, 1798 Ana Moreno, Evgenia Lefkaditou, Jean-Paul Robin, João Pereira, Angel F. Gon- zález, Sonia Seixas, Roger Villanueva, Graham J. Pierce, A. Louise Allcock, and Patrizia Jereb Common names Encornet (France), Καλαμάρι [calamary] (Greece), calamaro mediterraneo (Italy), lula vul- gar (Portugal), calamar común (Spain), European squid (UK) (Figure 11.1). Synonyms There are no synonyms for Loligo vulgaris. 11.1 Geographic distribution The European squid, Loligo vulgaris Lamarck, 1798, is found in the Northeast Atlantic from ca. 55°N to ca. 20°S and throughout the Mediterra- nean (Jereb et al., 2010). It is one of the most com- mon squids in the coastal waters of the Northeast Atlantic and the Mediterranean (Worms, 1983a). In the North Sea, its distribution extends from the northwest coast of Scotland, where it is occasion- ally reported (P. R. Boyle and G. J. Pierce, pers. comm.), to the Skagerrak and Kattegat, and a few old records from the western Baltic Sea (Grimpe, 1925; Tinbergen and Verwey, 1945) are sup- ported by more recent information (Muus, 1959 in Hornbörg, 2005). A record of one specimen la- belled Bergen (Norway; 60°23’N) is described in Figure 11.1. Loligo vulgaris. Dorsal Grieg (1933). view. From Muus (1959). Loligo vulgaris was not included by Massy (1928) in her list of the Cephalopoda of the Irish coast, and an early record of occurrence in the waters of the Isle of Man (Irish Sea; Moore, 1937, in Stephen, 1944) is doubtful. -

Feeding Ecology of Two Squid Species from the Western Mediterranean
Vol. 531: 207–219, 2015 MARINE ECOLOGY PROGRESS SERIES Published July 2 doi: 10.3354/meps11347 Mar Ecol Prog Ser Feeding ecology of two squid species from the western Mediterranean M. Valls1,*, M. Cabanellas-Reboredo2, I. Uranga1, A. Quetglas1 1Instituto Español de Oceanografía, Centre Oceanogràfic de les Balears, Moll de Ponent s/n, Apdo. 291, 07015 Palma, Spain 2Instituto Mediterráneo de Estudios Avanzados, IMEDEA (CSIC-UIB), C/Miquel Marqués 21, 07190 Esporles, Illes Balears, Spain ABSTRACT: The squid Loligo vulgaris (LV) and L. forbesii (LF) are 2 cephalopod species occur- ring in the Atlantic Ocean and the Mediterranean Sea. Bathymetric segregation allows the co- existence of both species, with LV preferentially inhabiting the shallow shelf and LF living on the shelf-break and upper slope grounds. In this paper, the feeding habits of LV and LF were studied for the first time in the Mediterranean, by means of stomach content analysis (1452 and 900 indi- viduals of LV and LF, respectively). The main objective was to determine the diet of both species, analysing temporal and ontogenetic diet changes and inferring predator−prey interactions. Fish were by far the most important prey in both squid, followed by crustaceans and cephalopods. Prey composition revealed the bathymetric segregation of both species in the Mediterranean. Whereas LV preferentially consumed typical coastal species of sparids and gobiids, LF preyed on slope inhabitants such as myctophids and euphausiids. Ontogenetic shifts of diet occurred in both squid, but took place at contrasting sizes, suggesting that the factors triggering them might be species- specific. The diet of small-sized LV individuals was more dependent on bottom-living organisms than in large individuals, which preyed mainly on benthopelagic fish. -

Environmental Determinants of Latitudinal Size-Trends in Cephalopods
Vol. 464: 153–165, 2012 MARINE ECOLOGY PROGRESS SERIES Published September 19 doi: 10.3354/meps09822 Mar Ecol Prog Ser Environmental determinants of latitudinal size-trends in cephalopods Rui Rosa1,*, Liliana Gonzalez2, Heidi M. Dierssen3, Brad A. Seibel4 1Laboratório Marítimo da Guia, Centro de Oceanografia, Faculdade de Ciências da Universidade de Lisboa, Av. Nossa Senhora do Cabo, 939, 2750-374 Cascais, Portugal 2Department of Computer Science and Statistics, University of Rhode Island, 9 Greenhouse Road, Kingston, Rhode Island 02881, USA 3Department of Marine Sciences, University of Connecticut, 1080 Shennecossett Road, Groton, Connecticut 06340-6048, USA 4Department of Biological Sciences, University of Rhode Island, 100 Flagg Road, Kingston, Rhode Island 02881, USA ABSTRACT: Understanding patterns of body size variation is a fundamental goal in ecology, but although well studied in the terrestrial biota, little is known about broad-scale latitudinal trends of body size in marine fauna and much less about the factors that drive them. We conducted a com- prehensive survey of interspecific body size patterns in coastal cephalopod mollusks, covering both hemispheres in the western and eastern Atlantic. We investigated the relationship between body size and thermal energy, resource and habitat availability and depth ranges. Both latitude and depth range had a significant effect on maximum body size in each of the major cephalopod groups (cuttlefishes, squids and octopuses). We observed significant negative associations between sea surface temperature (SST) and body size. No consistent relationships between body size and either net primary productivity (NPP), habitat extent (shelf area) or environmental varia- tion (range of SST and NPP) were found. -

Gastronomy and Gastrophysics of Danish Squid
Downloaded from orbit.dtu.dk on: Oct 07, 2021 Squids of the North: Gastronomy and gastrophysics of Danish squid Faxholm, Peter Lionet; Schmidt, Charlotte Vinther; Brønnum, Louise Beck; Sun, Yi Ting; Clausen, Mathias P.; Flore, Roberto; Olsen, Karsten Bæk; Mouritsen, Ole G. Published in: International Journal of Gastronomy and Food Science Link to article, DOI: 10.1016/j.ijgfs.2018.11.002 Publication date: 2018 Document Version Peer reviewed version Link back to DTU Orbit Citation (APA): Faxholm, P. L., Schmidt, C. V., Brønnum, L. B., Sun, Y. T., Clausen, M. P., Flore, R., Olsen, K. B., & Mouritsen, O. G. (2018). Squids of the North: Gastronomy and gastrophysics of Danish squid. International Journal of Gastronomy and Food Science, 14, 66-76. https://doi.org/10.1016/j.ijgfs.2018.11.002 General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Users may download and print one copy of any publication from the public portal for the purpose of private study or research. You may not further distribute the material or use it for any profit-making activity or commercial gain You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Author’s Accepted Manuscript Squids of the North: Gastronomy and gastrophysics of Danish squid Peter Lionet Faxholm, Charlotte Vinther Schmidt, Louise Beck Brønnum, Yi-Ting Sun, Mathias P. -

Patterns of Investment in Reproductive and Somatic Tissues in the Loliginid Squid Loligo Forbesii and Loligo Vulgaris in Iberian and Azorean Waters
Hydrobiologia (2011) 670:201–221 DOI 10.1007/s10750-011-0666-8 ECOSYSTEMS AND SUSTAINABILITY Patterns of investment in reproductive and somatic tissues in the loliginid squid Loligo forbesii and Loligo vulgaris in Iberian and Azorean waters Jennifer M. Smith • Graham J. Pierce • Alain F. Zuur • Helen Martins • M. Clara Martins • Filipe Porteiro • Francisco Rocha Published online: 5 April 2011 Ó Springer Science+Business Media B.V. 2011 Abstract The veined squid, Loligo forbesii, is found fishery landings data from 1990 to 1992 and employs throughout the northeast Atlantic, including the waters additive modelling to examine the relationships off the Iberian Peninsula, and is a socio-economically amongst somatic growth, season and gonad growth, important cephalopod species, sustaining several in an attempt to determine the relative importance of small-scale commercial and local artisanal fisheries. intrinsic (e.g. nutritional state and body size) and This study uses Iberian and Azorean trawl survey and extrinsic (temperature and daylight) factors which contribute to maturation in L. forbesii. We compare the results with those from a comparative analysis of Guest editors: Graham J. Pierce, Vasilis D. Valavanis, contemporaneous data on Loligo vulgaris from the M. Begon˜a Santos & Julio M. Portela / Marine Ecosystems and Sustainability Iberian coast, and with a re-analysis of previously published results for L. forbesii in Scottish waters. J. M. Smith (&) Á G. J. Pierce Reproductive organ weight in both sexes of School of Biological Sciences (Zoology), University of L. forbesii from all ports shows seasonal patterns with Aberdeen, Tillydrone Avenue, Aberdeen AB24 2TZ, UK e-mail: [email protected] a fall/winter peak in maturation, as is expected with the animals’ year-long life cycle.