Medication List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
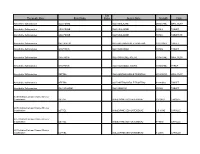
Therapeutic Class Brand Name P a Status Generic
P A Therapeutic Class Brand Name Status Generic Name Strength Form Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 250MG/5ML ORAL SUSP Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 500MG TABLET Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 500MG TABLET DR Absorbable Sulfonamides BACTRIM DS SULFAMETHOXAZOLE/TRIMETHO 800-160MG TABLET Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE 500MG TABLET Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE ACETYL 500MG/5ML ORAL SUSP Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE ACETYL 500MG/5ML SYRUP Absorbable Sulfonamides SEPTRA SULFAMETHOXAZOLE/TRIMETHO 200-40MG/5 ORAL SUSP Absorbable Sulfonamides SEPTRA SULFAMETHOXAZOLE/TRIMETHO 400-80MG TABLET Absorbable Sulfonamides SULFADIAZINE SULFADIAZINE 500MG TABLET ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 10-20MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 2.5-10MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-10MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-20MG CAPSULE P A Therapeutic Class Brand Name Status Generic Name Strength Form ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-40MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 10-40MG CAPSULE Acne Agents, Systemic ACCUTANE ISOTRETINOIN 10MG CAPSULE Acne Agents, Systemic ACCUTANE ISOTRETINOIN 20MG CAPSULE Acne Agents, Systemic ACCUTANE -

(12) Patent Application Publication (10) Pub. No.: US 2012/0190743 A1 Bain Et Al
US 2012O190743A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0190743 A1 Bain et al. (43) Pub. Date: Jul. 26, 2012 (54) COMPOUNDS FOR TREATING DISORDERS Publication Classification OR DISEASES ASSOCATED WITH (51) Int. Cl NEUROKININ 2 RECEPTORACTIVITY A6II 3L/23 (2006.01) (75) Inventors: Jerald Bain, Toronto (CA); Joel CD7C 69/30 (2006.01) Sadavoy, Toronto (CA); Hao Chen, 39t. ii; C Columbia, MD (US); Xiaoyu Shen, ( .01) Columbia, MD (US) A6IPI/00 (2006.01) s A6IP 29/00 (2006.01) (73) Assignee: UNITED PARAGON A6IP II/00 (2006.01) ASSOCIATES INC., Guelph, ON A6IPI3/10 (2006.01) (CA) A6IP 5/00 (2006.01) A6IP 25/00 (2006.01) (21) Appl. No.: 13/394,067 A6IP 25/30 (2006.01) A6IP5/00 (2006.01) (22) PCT Filed: Sep. 7, 2010 A6IP3/00 (2006.01) CI2N 5/071 (2010.01) (86). PCT No.: PCT/US 10/48OO6 CD7C 69/33 (2006.01) S371 (c)(1) (52) U.S. Cl. .......................... 514/552; 554/227; 435/375 (2), (4) Date: Apr. 12, 2012 (57) ABSTRACT Related U.S. Application Data Compounds, pharmaceutical compositions and methods of (60) Provisional application No. 61/240,014, filed on Sep. treating a disorder or disease associated with neurokinin 2 4, 2009. (NK) receptor activity. Patent Application Publication Jul. 26, 2012 Sheet 1 of 12 US 2012/O190743 A1 LU 1750 15OO 1250 OOO 750 500 250 O O 20 3O 40 min SampleName: EM2OO617 Patent Application Publication Jul. 26, 2012 Sheet 2 of 12 US 2012/O190743 A1 kixto CFUgan <tro CFUgan FIG.2 Patent Application Publication Jul. -

FROM MELANCHOLIA to DEPRESSION a HISTORY of DIAGNOSIS and TREATMENT Thomas A
1 FROM MELANCHOLIA TO DEPRESSION A HISTORY OF DIAGNOSIS AND TREATMENT Thomas A. Ban International Network for the History of Neuropsychopharmacology 2014 2 From Melancholia to Depression A History of Diagnosis and Treatment1 TABLE OF CONTENTS Introduction 2 Diagnosis and classifications of melancholia and depression 7 From Galen to Robert Burton 7 From Boissier de Sauvages to Karl Kahlbaum 8 From Emil Kraepelin to Karl Leonhard 12 From Adolf Meyer to the DSM-IV 17 Treatment of melancholia and depression 20 From opium to chlorpromazine 21 Monoamine Oxidase Inhibitors 22 Monoamine Re-uptake Inhibitors 24 Antidepressants in clinical use 26 Clinical psychopharmacology of antidepressants 30 Composite Diagnostic Evaluation of Depressive Disorders 32 The CODE System 32 CODE –DD 33 Genetics, neuropsychopharmacology and CODE-DD 36 Conclusions 37 References 37 INTRODUCTION Descriptions of what we now call melancholia or depression can be found in many ancient documents including The Old Testament, The Book of Job, and Homer's Iliad, but there is virtually 1 The text of this E-Book was prepared in 2002 for a presentation in Mexico City. The manuscript was not updated. 3 no reliable information on the frequency of “melancholia” until the mid-20th century (Kaplan and Saddock 1988). Between 1938 and 1955 several reports indicated that the prevalence of depression in the general population was below 1%. Comparing these figures, as shown in table 1, with figures in the 1960s and ‘70s reveals that even the lowest figures in the psychopharmacological era (from the 1960s) are 7 to 10 times greater than the highest figures before the introduction of antidepressant drugs (Silverman 1968). -

Design, Synthesis, and Evaluation of Antiepileptic Compounds Based on Β-Alanine and Isatin
Design, Synthesis, and Evaluation of Antiepileptic Compounds Based on β-Alanine and Isatin by Robert Philip Colaguori A thesis submitted in conformity with the requirements for the degree of Master of Science Department of Pharmaceutical Sciences University of Toronto © Copyright by Robert Philip Colaguori, 2016 ii Design, Synthesis, and Evaluation of Antiepileptic Compounds Based on β-Alanine and Isatin Robert Philip Colaguori Master of Science Department of Pharmaceutical Sciences University of Toronto 2016 Abstract Epilepsy is the fourth-most common neurological disorder in the world. Approximately 70% of cases can be controlled with therapeutics, however 30% remain pharmacoresistant. There is no cure for the disorder, and patients affected are subsequently medicated for life. Thus, there is a need to develop compounds that can treat not only the symptoms, but also delay/prevent progression. Previous work resulted in the discovery of NC-2505, a substituted β-alanine with activity against chemically induced seizures. Several N- and α-substituted derivatives of this compound were synthesized and evaluated in the kindling model and 4-AP model of epilepsy. In the kindling model, RC1-080 and RC1-102 were able to decrease the mean seizure score from 5 to 3 in aged mice. RC1-085 decreased the interevent interval by a factor of 2 in the 4-AP model. Future studies are focused on the synthesis of further compounds to gain insight on structure necessary for activity. iii Acknowledgments First and foremost, I would like to thank my supervisor Dr. Donald Weaver for allowing me to join the lab as a graduate student and perform the work ultimately resulting in this thesis. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Transdermal Drug Delivery Device Including An
(19) TZZ_ZZ¥¥_T (11) EP 1 807 033 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61F 13/02 (2006.01) A61L 15/16 (2006.01) 20.07.2016 Bulletin 2016/29 (86) International application number: (21) Application number: 05815555.7 PCT/US2005/035806 (22) Date of filing: 07.10.2005 (87) International publication number: WO 2006/044206 (27.04.2006 Gazette 2006/17) (54) TRANSDERMAL DRUG DELIVERY DEVICE INCLUDING AN OCCLUSIVE BACKING VORRICHTUNG ZUR TRANSDERMALEN VERABREICHUNG VON ARZNEIMITTELN EINSCHLIESSLICH EINER VERSTOPFUNGSSICHERUNG DISPOSITIF D’ADMINISTRATION TRANSDERMIQUE DE MEDICAMENTS AVEC COUCHE SUPPORT OCCLUSIVE (84) Designated Contracting States: • MANTELLE, Juan AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Miami, FL 33186 (US) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • NGUYEN, Viet SK TR Miami, FL 33176 (US) (30) Priority: 08.10.2004 US 616861 P (74) Representative: Awapatent AB P.O. Box 5117 (43) Date of publication of application: 200 71 Malmö (SE) 18.07.2007 Bulletin 2007/29 (56) References cited: (73) Proprietor: NOVEN PHARMACEUTICALS, INC. WO-A-02/36103 WO-A-97/23205 Miami, FL 33186 (US) WO-A-2005/046600 WO-A-2006/028863 US-A- 4 994 278 US-A- 4 994 278 (72) Inventors: US-A- 5 246 705 US-A- 5 474 783 • KANIOS, David US-A- 5 474 783 US-A1- 2001 051 180 Miami, FL 33196 (US) US-A1- 2002 128 345 US-A1- 2006 034 905 Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

Design, Synthesis, Characterization and Computational Docking Studies of Novel Sulfonamide Derivatives
EXCLI Journal 2018;17:169-180 – ISSN 1611-2156 Received: October 17, 2017, accepted: January 29, 2017, published: February 01, 2018 Original article: DESIGN, SYNTHESIS, CHARACTERIZATION AND COMPUTATIONAL DOCKING STUDIES OF NOVEL SULFONAMIDE DERIVATIVES Hira Saleem1a, Arooma Maryam1a, Saleem Ahmed Bokhari1a, Ayesha Ashiq1, Sadaf Abdul Rauf2, Rana Rehan Khalid1, Fahim Ashraf Qureshi1b, Abdul Rauf Siddiqi1 ⃰ 1 Department of Biosciences, COMSATS Institute of Information Technology, Islamabad, Pakistan 2 Department of Computer Science, Fatima Jinnah Women University, The Mall, Rawalpindi a Equal contributions, co-first authors b Shared correspondence: [email protected] ⃰ corresponding author: Abdul Rauf Siddiqi, Department of Biosciences, COMSATS Institute of Information Technology, Park Road, Islamabad, Pakistan E-mail: [email protected], [email protected] http://dx.doi.org/10.17179/excli2017-886 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). ABSTRACT This study reports three novel sulfonamide derivatives 4-Chloro-N-[(4-methylphenyl) sulphonyl]-N-propyl ben- zamide (1A), N-(2-hydroxyphenyl)-4-methyl benzene sulfonamide (1B) and 4-methyl-N-(2-nitrophenyl) ben- zene sulfonamide (1C). The compounds were synthesised from starting material 4-methylbenzenesulfonyl chlo- ride and their structure was studied through 1H-NMR and 13C-NMR spectra. Computational docking was per- formed to estimate their binding energy against bacterial p-amino benzoic acid (PABA) receptor, the dihydrop- teroate synthase (DHPS). The derivatives were tested in vitro for their antimicrobial activity against Gram+ and Gram- bacteria including E. coli, B. subtilis, B. licheniformis and B. linen. 1A was found active only against B. -

A Class-Selective Immunoassay for Sulfonamides Residue Detection in Milk Using a Superior Polyclonal Antibody with Broad Specificity and Highly Uniform Affinity
Article A Class-Selective Immunoassay for Sulfonamides Residue Detection in Milk Using a Superior Polyclonal Antibody with Broad Specificity and Highly Uniform Affinity Chenglong Li 1, Xiangshu Luo 1, Yonghan Li 2, Huijuan Yang 1, Xiao Liang 3, Kai Wen 1, Yanxin Cao 1, Chao Li 1, Weiyu Wang 1, Weimin Shi 1, Suxia Zhang 1, Xuezhi Yu 1,* and Zhanhui Wang 1 1 Beijing Advanced Innovation Center for Food Nutrition and Human Health, College of Veterinary Medicine, China Agricultural University, Beijing Key Laboratory of Detection Technology for Animal- Derived Food Safety, Beijing Laboratory of Food Quality and Safety, 100193 Beijing, China; [email protected] (C.L.); [email protected] (X.L.); [email protected] (H.Y.); [email protected] (K.W.); [email protected] (Y.C.); [email protected] (C.L.); [email protected] (W.W.); [email protected] (W.S.); [email protected] (S.Z.); [email protected] (Z.W.) 2 Henan Animal Health Supervision Institute, 450008 Zhengzhou, China; [email protected] 3 College of Veterinary Medicine, Qingdao Agricultural University, 266109 Qingdao, China; [email protected] * Correspondence: [email protected]; Tel.: +86-10-62734565; Fax: +86-10-62731032 Academic Editors: Patricia Regal and Carlos M. Franco Received: 13 December 2018; Accepted: 24 January 2019; Published: 26 January 2019 Abstract: The development of multianalyte immunoassays with an emphasis on food safety has attracted increasing interest, due to its high target throughput, short detection time, reduced sample consumption, and low overall cost. In this study, a superior polyclonal antibody (pAb) against sulfonamides (SAs) was raised by using a bioconjugate of bovine serum albumin with a rationally designed hapten 4-[(4-aminophenyl) sulfonyl-amino]-2-methoxybenzoic acid (SA10-X). -

Dietary Supplements Compendium Volume 1
2015 Dietary Supplements Compendium DSC Volume 1 General Notices and Requirements USP–NF General Chapters USP–NF Dietary Supplement Monographs USP–NF Excipient Monographs FCC General Provisions FCC Monographs FCC Identity Standards FCC Appendices Reagents, Indicators, and Solutions Reference Tables DSC217M_DSCVol1_Title_2015-01_V3.indd 1 2/2/15 12:18 PM 2 Notice and Warning Concerning U.S. Patent or Trademark Rights The inclusion in the USP Dietary Supplements Compendium of a monograph on any dietary supplement in respect to which patent or trademark rights may exist shall not be deemed, and is not intended as, a grant of, or authority to exercise, any right or privilege protected by such patent or trademark. All such rights and privileges are vested in the patent or trademark owner, and no other person may exercise the same without express permission, authority, or license secured from such patent or trademark owner. Concerning Use of the USP Dietary Supplements Compendium Attention is called to the fact that USP Dietary Supplements Compendium text is fully copyrighted. Authors and others wishing to use portions of the text should request permission to do so from the Legal Department of the United States Pharmacopeial Convention. Copyright © 2015 The United States Pharmacopeial Convention ISBN: 978-1-936424-41-2 12601 Twinbrook Parkway, Rockville, MD 20852 All rights reserved. DSC Contents iii Contents USP Dietary Supplements Compendium Volume 1 Volume 2 Members . v. Preface . v Mission and Preface . 1 Dietary Supplements Admission Evaluations . 1. General Notices and Requirements . 9 USP Dietary Supplement Verification Program . .205 USP–NF General Chapters . 25 Dietary Supplements Regulatory USP–NF Dietary Supplement Monographs . -

High-Throughput Brain Activity Mapping and Machine Learning As a Foundation for Systems Neuropharmacology
High-throughput brain activity mapping and machine learning as a foundation for systems neuropharmacology Lin, Xudong; Duan, Xin; Jacobs, Claire; Ullmann, Jeremy; Chan, Chung-Yuen; Chen, Siya; Cheng, Shuk-Han; Zhao, Wen-Ning; Poduri, Annapurna; Wang, Xin; Haggarty, Stephen J.; Shi, Peng Published in: Nature Communications Published: 03/12/2018 Document Version: Final Published version, also known as Publisher’s PDF, Publisher’s Final version or Version of Record License: CC BY Publication record in CityU Scholars: Go to record Published version (DOI): 10.1038/s41467-018-07289-5 Publication details: Lin, X., Duan, X., Jacobs, C., Ullmann, J., Chan, C-Y., Chen, S., Cheng, S-H., Zhao, W-N., Poduri, A., Wang, X., Haggarty, S. J., & Shi, P. (2018). High-throughput brain activity mapping and machine learning as a foundation for systems neuropharmacology. Nature Communications, 9, [5142]. https://doi.org/10.1038/s41467-018-07289- 5 Citing this paper Please note that where the full-text provided on CityU Scholars is the Post-print version (also known as Accepted Author Manuscript, Peer-reviewed or Author Final version), it may differ from the Final Published version. When citing, ensure that you check and use the publisher's definitive version for pagination and other details. General rights Copyright for the publications made accessible via the CityU Scholars portal is retained by the author(s) and/or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Users may not further distribute the material or use it for any profit-making activity or commercial gain. -

"2-Oxo-1-Pyrrolidine Derivative and Its Pharmaceutical Uses"
(19) & (11) EP 1 452 524 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07D 207/27 (2006.01) A61K 31/4015 (2006.01) 14.10.2009 Bulletin 2009/42 A61P 25/08 (2006.01) (21) Application number: 04007878.4 (22) Date of filing: 21.02.2001 (54) "2-oxo-1-pyrrolidine derivative and its pharmaceutical uses" "2-Oxo-1-Pyrrolidinderivate und ihre pharmazeutische Verwendung" "Dérivé de 2-oxo-1-pyrrolidine et ses applications pharmaceutique" (84) Designated Contracting States: • Talaga, Patrice AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU 1170 Watermael-Boitsfort (BE) MC NL PT SE TR (74) Representative: UCB (30) Priority: 23.02.2000 GB 0004297 Intellectual Property c/o UCB S.A. (43) Date of publication of application: Intellectual Property Department 01.09.2004 Bulletin 2004/36 Allée de la Recherche 60 1070 Brussels (BE) (62) Document number(s) of the earlier application(s) in accordance with Art. 76 EPC: (56) References cited: 01925354.1 / 1 265 862 WO-A-99/13911 GB-A- 1 309 692 (73) Proprietor: UCB Pharma, S.A. • PROUS: "LEVETIRACETAM" DRUGS OF THE 1170 Brussels (BE) FUTURE, BARCELONA, ES, vol. 19, no. 2, 1994, pages 111-113, XP000908882 ISSN: 0377-8282 (72) Inventors: • FISCHER W ET AL: "Die Wirksamkeit von • Differding, Edmond Piracetam, Meclofenoxat und Vinpocetin in 1348 Ottignies-Louvain-La-Neuve (BE) verschiedenen Krampfmodellen bei der Maus" • Kenda, Benoît PHARMAZIE, VEB VERLAG VOLK UND 5080 Emines (BE) GESUNDHEIT. BERLIN, DD, vol. 46, no. -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG .