View Full Text-PDF
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

Success Story and Factors Affecting Level of Income Earned From
Bassa et al., Irrigat Drainage Sys Eng 2017, 6:1 ge Syst ina em a s r D E DOI: 10.4172/2168-9768.1000181 n & g i n n o e i t e a r i g n i r g r Irrigation & Drainage Systems Engineering I ISSN: 2168-9768 Research Article Open Access Success Story and Factors Affecting Level of Income Earned from Improved Potato Farming in Damot Sore Woreda, Wolaita and Southern Ethiopia Bassa Z*, Abera A, Zeleke B, Alemu M, Bashe A and Areka MS Southern Agricultural Research Institute, Areka Agricultural Research Center, Areka, Ethiopia Abstract Increasing production and productivity of crop farming ,improving income of resource poor farmers and thereby enabling the producers to build assed in Southern Ethiopia in General and Damot Sore Woreda in particular require some form of transformation of the subsistence, low-input and low-productivity farming systems to full agricultural packages utilization and awareness creation. The study was employed in Irish AID Operational Research and Technology Dissemination Project (ORTDP) areas of Areka mandate. This study was undertaken to analyse factors affecting level of income earned from Potato and summarize benefits of utilizing improved potato variety and full agricultural packages in the district. A multi-stage sampling technique was used to select 80 sample households from two sample kebele. In the study, both primary and secondary data sources were used. Simple Linear Regression Model was employed to identify factors affecting level of income earned from improved potato production by resource poor farmers in the district. Results showed that using improved potato variety increase the production and productivity of the specific commodity and there help the resource poor farmers to build asset. -

Protecting Land Tenure Security of Women in Ethiopia: Evidence from the Land Investment for Transformation Program
PROTECTING LAND TENURE SECURITY OF WOMEN IN ETHIOPIA: EVIDENCE FROM THE LAND INVESTMENT FOR TRANSFORMATION PROGRAM Workwoha Mekonen, Ziade Hailu, John Leckie, and Gladys Savolainen Land Investment for Transformation Programme (LIFT) (DAI Global) This research paper was created with funding and technical support of the Research Consortium on Women’s Land Rights, an initiative of Resource Equity. The Research Consortium on Women’s Land Rights is a community of learning and practice that works to increase the quantity and strengthen the quality of research on interventions to advance women’s land and resource rights. Among other things, the Consortium commissions new research that promotes innovations in practice and addresses gaps in evidence on what works to improve women’s land rights. Learn more about the Research Consortium on Women’s Land Rights by visiting https://consortium.resourceequity.org/ This paper assesses the effectiveness of a specific land tenure intervention to improve the lives of women, by asking new questions of available project data sets. ABSTRACT The purpose of this research is to investigate threats to women’s land rights and explore the effectiveness of land certification interventions using evidence from the Land Investment for Transformation (LIFT) program in Ethiopia. More specifically, the study aims to provide evidence on the extent that LIFT contributed to women’s tenure security. The research used a mixed method approach that integrated quantitative and qualitative data. Quantitative information was analyzed from the profiles of more than seven million parcels to understand how the program had incorporated gender interests into the Second Level Land Certification (SLLC) process. -

Prevalence and Economic Significance of Hydatidosis in Bovine Slaughtered at Kindo Koysha Woreda Municipality Abattoir, Ethiopia
International Journal of Research Studies in Biosciences (IJRSB) Volume 6, Issue 7, 2018, PP 31-37 ISSN No. (Online) 2349-0365 DOI: http://dx.doi.org/10.20431/2349-0365.0607005 www.arcjournals.org Prevalence and Economic Significance of Hydatidosis in Bovine Slaughtered at Kindo Koysha Woreda Municipality Abattoir, Ethiopia Dawit Dana Wolaita sodo univeresity, School of veterinary medicine *Corresponding Author: Dawit Dana, Wolaita sodo univeresity, School of veterinary medicine Abstract: A cross-sectional study of bovine hydatidosis was conducted in Bele Municipality Abattoir from December, 2016 to August, 2017 to estimate the prevalence and economic impact of hydatidosis in cattle slaughtered at Bele Municipality Abattoir. Abattoir survey of hydatidosis was conducted during routine meat inspection activity on randomly selected 384 cattle encountered at Bele Municipality Abattoir. Ante-mortem examination was conducted to note the breed, age, sex and body condition of study animals. These animals were given number in order to identify them during the postmortem examination. Post mortem examination was conducted to note the presence of hydatid cysts. A total of 56 (14.57%) cattle were affected with hydatid cyst. The study shows that there was significant variation in the prevalence of hydatid cyst in different peasant association (p<0.05). However, no such association was observed in prevalence of hydatid cysts according to sex, age, breed and body condition of slaughtered animals (p=0.499, p=0.086, p=0.613, p=0.140, respectively). The study period of the economic loss due to organ condemnation associated to bovine hydatidosis at the abattoir was estimated 6,720.00 Ethiopian Birr. -

Ethiopia: Administrative Map (August 2017)
Ethiopia: Administrative map (August 2017) ERITREA National capital P Erob Tahtay Adiyabo Regional capital Gulomekeda Laelay Adiyabo Mereb Leke Ahferom Red Sea Humera Adigrat ! ! Dalul ! Adwa Ganta Afeshum Aksum Saesie Tsaedaemba Shire Indasilase ! Zonal Capital ! North West TigrayTahtay KoraroTahtay Maychew Eastern Tigray Kafta Humera Laelay Maychew Werei Leke TIGRAY Asgede Tsimbila Central Tigray Hawzen Medebay Zana Koneba Naeder Adet Berahile Region boundary Atsbi Wenberta Western Tigray Kelete Awelallo Welkait Kola Temben Tselemti Degua Temben Mekele Zone boundary Tanqua Abergele P Zone 2 (Kilbet Rasu) Tsegede Tselemt Mekele Town Special Enderta Afdera Addi Arekay South East Ab Ala Tsegede Mirab Armacho Beyeda Woreda boundary Debark Erebti SUDAN Hintalo Wejirat Saharti Samre Tach Armacho Abergele Sanja ! Dabat Janamora Megale Bidu Alaje Sahla Addis Ababa Ziquala Maychew ! Wegera Metema Lay Armacho Wag Himra Endamehoni Raya Azebo North Gondar Gonder ! Sekota Teru Afar Chilga Southern Tigray Gonder City Adm. Yalo East Belesa Ofla West Belesa Kurri Dehana Dembia Gonder Zuria Alamata Gaz Gibla Zone 4 (Fantana Rasu ) Elidar Amhara Gelegu Quara ! Takusa Ebenat Gulina Bugna Awra Libo Kemkem Kobo Gidan Lasta Benishangul Gumuz North Wello AFAR Alfa Zone 1(Awsi Rasu) Debre Tabor Ewa ! Fogera Farta Lay Gayint Semera Meket Guba Lafto DPubti DJIBOUTI Jawi South Gondar Dire Dawa Semen Achefer East Esite Chifra Bahir Dar Wadla Delanta Habru Asayita P Tach Gayint ! Bahir Dar City Adm. Aysaita Guba AMHARA Dera Ambasel Debub Achefer Bahirdar Zuria Dawunt Worebabu Gambela Dangura West Esite Gulf of Aden Mecha Adaa'r Mile Pawe Special Simada Thehulederie Kutaber Dangila Yilmana Densa Afambo Mekdela Tenta Awi Dessie Bati Hulet Ej Enese ! Hareri Sayint Dessie City Adm. -

An Assessment of Rural Households Livelihood Assets of Wolaita Zone, Ethiopia
Research on Humanities and Social Sciences www.iiste.org ISSN 2224-5766 (Paper) ISSN 2225-0484 (Online) Vol.10, No.5, 2020 An Assessment of Rural Households Livelihood Assets of Wolaita Zone, Ethiopia Deneke Dana Dabara (PhD) Economic Geography, Wolaita Sodo University, Ethiopia Abstract The aim of this study was to assess existing rural household’s livelihood assets in Wolaita zone, Ethiopia. The demographic and socio-economic data were collected from 300 randomly selected rural households. The basic data for the study were obtained from both primary and secondary sources. The collected data were analysed by using both qualitative and quantitative methods. The study revealed land is one of the basic economic assets of and livelihood sources for agrarian rural people but the size of the plots is so small in the study area. The findings concerning the livelihood assets that had been developed were presented in line with the three livelihood assets, namely human, natural, and financial capital, all of which were shown to have negative results. The above- mentioned livelihood assets in the study area were weak. From the human assets section, 41percent of the study population had not achieved a school educational qualification. About half percent of respondents said their soil was not fertile from the physical assets perspective. A large number of respondents did not practise saving on account of their low daily-income status. Thus concerning body including government should strongly work on the discussed rural assets built up to minimize the rural household’s livelihood problems in study area. Keywords: Wolaita, rural, assets, human, natural, financial, households, livelihood DOI: 10.7176/RHSS/10-5-01 Publication date: March 31 st 2020 1.INTRODUCTION Of all the less-developed regions of the world, Sub-Saharan Africa is the most severely affected region in terms of livelihood problems. -

Ethiopia: SNNP Region Administrative Map (As of 15 Aug 2017)
Ethiopia: SNNP region administrative map (as of 15 Aug 2017) ! ! ! ! ! ! ! ! ! Suten ! ! ! ! ! ! Inge Sodo ! ! !Bui ! ! WelikiteKebena Abeshege ! Kokir Gedbano ! ! Kela ! ! Muhur Na Ak!lil ! Gubire ! ! ! Cheha Agena ! Imdibir! ! Ezha Me!skan ! ! Inseno ! Gonichire ! ! ! Kibet Qewaqoto! Koshe ! ! ! ! ! ! ! Enemorina Eaner Alicho Woriro ! Gumer Mareko ! Selti ! ! Areket Alkeso town ! ! ! ! ! ! Geta Kose Tora ! Fofa ! Werabe ! ! ! Dinkela ! ! Sayilem! ! ! ! ! Yadota Geja Endiguagn Yem SP Woreda ! Dalocha ! Misrak Azenet Berbere ! ! ! ! Misha !LERA Dalocha Masha ! Wilb!areg Gibe ! ! Mierab Azenet Berbere ! ! Lanfero ! Homec!ho ! ! Fonqo town ! Mito ! GAMBELA Gesha (Deka) Kondo GECHA TOWN ! Analemmo ! ! !Deka ! Doesha !Belesa town ! Alem Gebeya Anderacha Getawa Gembora ! ! Limu ! ! Bonosha Sankura ! ! ! Lisana town Jajira Shashogo Gimbichu! ! Hufa ! ! ! Diri Soro ! Gojeb Bita (Big) Gimbo Doya Gena Jacho A!nigach!a ! Alaba SP Woreda ! ! ! Daniboya Wishiwishi Dune Kulito ! Kaka Idget ! Bita Genet ! OROMIA Kelata Mudula Hobichaka ! ! Bonga ! ! ! ! ! Yeki ! Menjiwo ! Chena Tembaro Ke!diada Gambela TEPI TOWN Hadero !TubitoKacha Bira ! ! ! !Adilo Chda Idge T!unito ! Legend WACHA ! ! Terche Misrak Badawacho ! Gena Bosa Chiri BOMIBE 01 ! ! ! ! !Karewo ! Mierab Badawacho ! Ameya P ! Tocha Tocha Edget Boloso Bombe Sheka Tulo ! Regional capital ! Waka ! Semen Bench Alem Gena ! ! ! ! Mehal Sheko Mareka Boloso SoreDamot Pulasa Hawassa Zuria PWondo-Ge! net Gesa ! ! Shanito Hawasa Town ! ! ! ! Shama Chuko Shay Bench ! Bitena Town Mizan Aman ! ! Tula ! Damot -
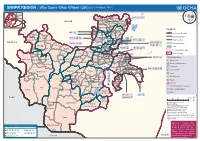
SNNPR REGION : Who Does What Where (3W) (As of 01 March 2012)
(as of 01 March 2012) SNNPR REGION : Who Does What Where (3W) Tigray Afar Amhara Sodo! ECS: a cç Benshangul Gumuz KebenaKokir Gedbano Dire Dawa Abeshege Addis Ababa Hareri Gambela Oromia Oromia Muhur Na Aklil Somali Cheha SNNPR Gurage Ezha Meskan Alicho Woriro Enemorina Eaner Gumer Selti Mareko Yem Geta Selti Legend Sayilem ! IRC: ç Endiguagn Dalocha Yem SP Wor!eda International Boundary Masha Gibe Misha Wilbareg Lanfero Regional Boundary Sheka Gesha (Deka) ECS: ah ç HadiyaAnalemmo ! Anderacha Getawa Gembora ! Sankura LVIA: a 4 l Zonal Boundary ! Limu Gambella Shash! ogo LVIA: a 4 l Plan Int.: : h Soro Woreda Boundary Gimbo Anigacha Alaba SP Woreda SC UK: h Bita (Big) ! Lake Dune Daniboy! a Alaba KT Plan Int.: h IMC: î h ç Yeki Chena Menjiwo Tembaro Keffa Kacha BiraKediada Gambela No Intervention/No Data Gena Bosa Misrak Badawacho Tocha Boloso Bom! be Other Region Sheka Tulo Wondo-Genet Semen Bench Boloso sore Awassa Zuria Mareka A! wasa Town Dawro Damot Gale Plan Int.: d Clusters/Sectors Ela (Konta) SP Woreda Kindo Koysha Diguna Fango ! Malga Gurafereda Debub BenchShay Bench Cheta Boricha Agriculture Decha Esira Damot Sore a Konta Loma Bosa Sodo ZuriaDamot Weydie Shebe DinoGorche Wolayita Dale : Disaster Management Menit Goldiye Kindo Dida Ofa Humbo Wonosho Arbe Gonna d Education ! Loka-Abaya ! Bursa Sidama ACF: aîlf Chuko 4 Environment Melekoza Kucha Boreda Bensa Menit Shasha ! Hulla Denibu Gofa Dara Bona Zu! ria ç Chire î Food Dila Zuria Bero BasketoGeze Gofa h Aroresa l Food Security Zala Mirab Abaya Wenago Basketo SP Woreda DaramaloDita -

Guide 2000 English.Pub
1 MAY 2008 AWASSA 2 Table of Contents Page N0 • Introduction 1 Part I Location and Administrative Division—- - - - - - - - - - - - - - 2 • Topography —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2 • Climate —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - • Soil Resource —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3 • Land use and land cover —- - - - - - - - - - - - - - - - - - - - - - - - - 3 • Water bodies —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - • Forest Resource —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5 • Wild life resource —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5 • Tourist Attraction and potential —- - - - - - - - - - - - - - - - - - - 6 • Population —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 7 • Ethnic Composition —- - - - - - - - - - - - - - - - - - - - - - - - - 8 • Urbanization —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8 • Agriculture —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8 • Livestock resource —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 9 • Fisher —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 9 • Hides and skins production and market —- - - - - - - - - - - - - - 9 • Apiculture —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 9 • Industry —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 9 • Education—- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -

Drought in Ethiopia – 16Th May 2008
United Nations Nations Unies Office for the Coordination of Bureau de Coordination des Humanitarian Affairs in Ethiopia Affaires Humanitaires au Ethiopie Website: Website: http://ochaonline.un.org/ethiopia http://ochaonline.un.org/ethiopia SITUATION REPORT: DROUGHT IN ETHIOPIA – 16TH MAY 2008 Highlights: • WFP estimated number of those in need of emergency food relief: 3.4 million people from July to September and likely to increase. • Estimated population at risk from AWD is 3,045,095 in Arsi and West Arsi and 2,077,655 in West Showa. • UNICEF estimates that in 98 woredas in SNNPR and Oromiya the number of children under five years of age in need of immediate therapeutic care is in excess of 61,000. Of those, 33,000 children living in 55 woredas covered by NGO activity are in need of treatment for Severe Acute Malnutrition. And as many as 28,000 living in 43 woredas not covered by NGO activity are also in need of treatment for Severe Acute Malnutrition. • UNICEF additionally estimates that due to the drought as many as 6 million children under five are currently at risk of acute malnutrition. • Current population in therapeutic feeding centres in SNNPR and Oromiya: 7000 and rising. • Malnutrition in SNNPR and Oromiya continue to increase at alarming rates. Kwashiorkor and other malnutrition-related diseases are prevalent in hotspot areas. • UNICEF and WFP resources to address both severe malnutrition and moderate malnutrition in affected areas are severely strained. Both blended food and Plumpy’Nut are in demand yet stocks are very limited. WFP facing immediate pipeline break of 40,000 MT of Cory-Soya Blend (CSB) for relief and targeted supplementary feeding. -

Ethiopia 2015 Fpwatch Reference Document
FPwatch Study Reference Document Federal Democratic Republic of Ethiopia Outlet Survey 2015-16 www.FPwatch.info Copyright © Population Services International (PSI). All rights reserved. Released April 2016 Suggested citation FPwatch Group. (2016). FPwatch Study Reference Document: The Federal Democratic Republic of Ethiopia 2015. Washington DC: PSI. Contact Tarryn Haslam Dr. Woldemariam Girma Deputy Director, Malaria & Child Principal Investigator – Population Services International (PSI) Survival Department Ethiopia PSI | 1120 19th St NW Suit 600 Addis Ababa, Bole Sub-City, Kebele 03/05 Washington DC 20036 Bole Medahneyalem Church Area [email protected] Addis Ababa, Ethiopia [email protected] Acknowledgements FPwatch is funded by the Bill and Melinda Gates Foundation. This study was implemented by Population Services International. PSI-Ethiopia Field Supervisors Field Team Members Dr. Woldemariam Girma Akele Kubi Abdulahi Ahmed Leul Haile Donato Gulino Berhanu Yitayew Admasu Daka Mekoya Tarekegn Elias Legesse Dereje Bayissa Ali Jemal Mesfin Agachew Girma Tadesse Dereje Dechasa Anwar Jibril Mesfin Girma Habtamu Tamene Desalegn Geleta Asfaw Oljira Migret Ayalew Desalegn Tadesse Asmamaw Haile Mulunesh Amenta Alliance for Better Health Getachew Tilahun Aster Tegene Nathnael Senbetu Fekadu Dubi Hanna Mersha Bedilu Alemayehu Nefise Nuriye Dr. Marcos Feleke Henok Demissie Beruh Alemayehu Omar Ahmed Eyob Kifle Kasegn Tibebu Biruk Amare Rehel Tedele Dr. Mengistu Tafesse Megnistu Yilma Biruk Taye Rakeb Abebaw Tsedeke Wolde Chaltu Baja Sabit -

Gibe III-Sodo 400 Kv Power Transmission Lines Project
FEDERAL DEMOCRATIC REPUBLIC OF ETHIOPIA ETHIOPIAN ELECTRIC POWER CORPORATION (EEPCo) Gibe III-Sodo 400 kV Power Transmission Lines Project RESETTLEMENT ACTION PLAN EEPCo, Environmental Monitoring Unit (EMU) E-mail [email protected] January 2009 Table of Contents Page EXECUTIVE SUMMARY i 1. INTRODUCTION ............................................................................................................ 1 1.1 Back Ground of the Project ........................................................................................ 1 1.2 Objectives of the Resettlement Plan ........................................................................... 1 1.3 Methodology .............................................................................................................. 2 1.3.1 Review Literature ................................................................................................ 2 1.3.2 Census and Socio-Economic Survey .................................................................. 2 1.3.3 Public Consultation ............................................................................................. 2 1.4 Assessment Team ....................................................................................................... 2 2. OVERVIEW OF THE PROJECT .................................................................................... 3 2.1 Description of the Project Components ..................................................................... 3 2.1.1 Towers................................................................................................................