Review Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

35 Disorders of Purine and Pyrimidine Metabolism
35 Disorders of Purine and Pyrimidine Metabolism Georges van den Berghe, M.- Françoise Vincent, Sandrine Marie 35.1 Inborn Errors of Purine Metabolism – 435 35.1.1 Phosphoribosyl Pyrophosphate Synthetase Superactivity – 435 35.1.2 Adenylosuccinase Deficiency – 436 35.1.3 AICA-Ribosiduria – 437 35.1.4 Muscle AMP Deaminase Deficiency – 437 35.1.5 Adenosine Deaminase Deficiency – 438 35.1.6 Adenosine Deaminase Superactivity – 439 35.1.7 Purine Nucleoside Phosphorylase Deficiency – 440 35.1.8 Xanthine Oxidase Deficiency – 440 35.1.9 Hypoxanthine-Guanine Phosphoribosyltransferase Deficiency – 441 35.1.10 Adenine Phosphoribosyltransferase Deficiency – 442 35.1.11 Deoxyguanosine Kinase Deficiency – 442 35.2 Inborn Errors of Pyrimidine Metabolism – 445 35.2.1 UMP Synthase Deficiency (Hereditary Orotic Aciduria) – 445 35.2.2 Dihydropyrimidine Dehydrogenase Deficiency – 445 35.2.3 Dihydropyrimidinase Deficiency – 446 35.2.4 Ureidopropionase Deficiency – 446 35.2.5 Pyrimidine 5’-Nucleotidase Deficiency – 446 35.2.6 Cytosolic 5’-Nucleotidase Superactivity – 447 35.2.7 Thymidine Phosphorylase Deficiency – 447 35.2.8 Thymidine Kinase Deficiency – 447 References – 447 434 Chapter 35 · Disorders of Purine and Pyrimidine Metabolism Purine Metabolism Purine nucleotides are essential cellular constituents 4 The catabolic pathway starts from GMP, IMP and which intervene in energy transfer, metabolic regula- AMP, and produces uric acid, a poorly soluble tion, and synthesis of DNA and RNA. Purine metabo- compound, which tends to crystallize once its lism can be divided into three pathways: plasma concentration surpasses 6.5–7 mg/dl (0.38– 4 The biosynthetic pathway, often termed de novo, 0.47 mmol/l). starts with the formation of phosphoribosyl pyro- 4 The salvage pathway utilizes the purine bases, gua- phosphate (PRPP) and leads to the synthesis of nine, hypoxanthine and adenine, which are pro- inosine monophosphate (IMP). -

Drug Antioxidant Effects a Basis for Drug Selection?
REVIEW ARTI LE Drugs 42 (4): 569-605, 1991 00 12-6667/ 91/ 00 I0-0569/ $ 18.50/0 © Adis International Limited. All rights reserved. DRU161 Drug Antioxidant Effects A Basis for Drug Selection? Barry Halliwell Pulmonary Medicine, UC Davis Medical Center, Sacramento , California, USA Contents 570 Summary 570 I. What is a Free Radical? 570 1.1 Definition of a Free Radical 57/ 1.2 Hydroxyl Radical 572 1.3 Formation of Oxygen Radicals In Vivo 574 1.4 Reactive Oxygen Species 575 1.5 Transition Metal Ions and Free Radical Reactions 575 1.6 Antioxidant Defence In Vivo: Intracellular 576 1.7 Antioxidant Defence In Vivo: Extracellular 579 2. Free Radical Damage to Cells and the Protective Ability of Antioxidants 579 2.1 Mechanisms of Cell Damage: The Molecular Targets 58/ 2.2 How to Characterise an Antioxidant 5 2 3. The Role of Free Radicals in Human Disease 582 3.1 Causation of Disease 582 3.2 A Significant Consequence of Disease Pathology 584 3.3 A General Consequence of Tissue Injury 585 3.4 What Can We Expect from Antioxidant Therapy? 586 586 4. Antioxidants in Disease Therapy 586 4.1 Use of Naturally Occurring Antioxidants and Derived Compounds 587 4.1.1 Superoxide Dismutase 587 4.1.2 Superoxide Dismutase 'Mimics' 588 4.1.3 a-Tocopherol 589 4.1.4 Ascorbic Acid (Vitamin C) 589 4.1.5 Adenosine 589 4.1.6 Lactoferrin 590 4.1.7 Nicotinamide 59/ 4.1.8 Glutathione and its Precursors 59/ 4.1.9 Carotenoids 59/ 4.2 'Synthetic' Antioxidants 59/ 4.2.1 Mercaptopropionylglycine and Other Thiols 592 4.2.2 Ebselen: A Glutathione Peroxidase Mimic? 593 4.2.3 Allopurinol, Oxypurinol and Other Xanth ine Oxidase Inhibitors 593 4.2.4 Inhibitors of the Generation of Reactive Oxygen Species by Phagocytes 595 4.2.5 Chelating Agents: Deferoxamine 595 4.2.6 Other Iron Chelators: Hydroxypyridones 596 4.2.7 Other Iron Chelators: ICRF-187 and the 'Lazaroids' 4.2.8 Probucol and Other Chain-Breaking Antioxidants 570 Drugs 42 (4) 1991 596 5. -
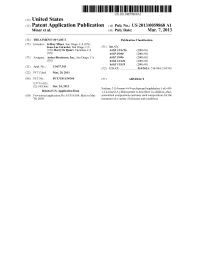
(12) Patent Application Publication (10) Pub. No.: US 2013/0059868 A1 Miner Et Al
US 2013 0059868A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0059868 A1 Miner et al. (43) Pub. Date: Mar. 7, 2013 (54) TREATMENT OF GOUT Publication Classification (75) Inventors: Jeffrey Miner, San Diego, CA (US); Jean-Luc Girardet, San Diego, CA (51) Int. Cl. (US); Barry D. Quart, Encinitas, CA A613 L/496 (2006.01) (US) A6IP 29/00 (2006.01) (73) Assignee: Ardea Biociences, Inc., San Diego, CA A6IP 9/06 (2006.01) (US) A613 L/426 (2006.01) A 6LX3/59 (2006.01) (21) Appl. No.: 13/637,343 (52) U.S. Cl. ...................... 514/262.1: 514/384: 514/365 (22) PCT Fled: Mar. 29, 2011 (86) PCT NO.: PCT/US11A3O364 (57) ABSTRACT S371 (c)(1), (2), (4) Date: Oct. 24, 2012 Sodium 2-(5-bromo-4-(4-cyclopropyl-naphthalen-1-yl)-4H Related U.S. Application Data 1,2,4-triazol-3-ylthio)acetate is described. In addition, phar (60) Provisional application No. 61/319,014, filed on Mar. maceutical compositions and uses Such compositions for the 30, 2010. treatment of a variety of diseases and conditions. Patent Application Publication Mar. 7, 2013 Sheet 1 of 10 US 2013/00598.68A1 FIGURE 1 S. C SOC 55000 40 S. s 3. s Patent Application Publication Mar. 7, 2013 Sheet 2 of 10 US 2013/00598.68A1 ~~~::CC©>???>©><!--->?©><??--~~~~~·%~~}--~~~~~~~~*~~~~~~~~·;--~~~~~~~~~;~~~~~~~~~~}--~~~~~~~~*~~~~~~~~;·~~~~~ |×.> |||—||--~~~~ ¿*|¡ MSU No IL-1ra MSU50 IL-1ra MSU 100 IL-1ra MSU500 IL-1ra cells Only No IL-1 ra Patent Application Publication Mar. 7, 2013 Sheet 3 of 10 US 2013/00598.68A1 FIGURE 3 A: 50000 40000 R 30000 2 20000 10000 O -7 -6 -5 -4 -3 Lesinurad (log)M B: Lesinurad (log)M Patent Application Publication Mar. -

PHARMACEUTICAL APPENDIX to the TARIFF SCHEDULE 2 Table 1
Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

(12) United States Patent (10) Patent No.: US 9,642,912 B2 Kisak Et Al
USOO9642912B2 (12) United States Patent (10) Patent No.: US 9,642,912 B2 Kisak et al. (45) Date of Patent: *May 9, 2017 (54) TOPICAL FORMULATIONS FOR TREATING (58) Field of Classification Search SKIN CONDITIONS CPC ...................................................... A61K 31f S7 (71) Applicant: Crescita Therapeutics Inc., USPC .......................................................... 514/171 Mississauga (CA) See application file for complete search history. (72) Inventors: Edward T. Kisak, San Diego, CA (56) References Cited (US); John M. Newsam, La Jolla, CA (US); Dominic King-Smith, San Diego, U.S. PATENT DOCUMENTS CA (US); Pankaj Karande, Troy, NY (US); Samir Mitragotri, Santa Barbara, 5,602,183 A 2f1997 Martin et al. CA (US); Wade A. Hull, Kaysville, UT 5,648,380 A 7, 1997 Martin 5,874.479 A 2, 1999 Martin (US); Ngoc Truc-ChiVo, Longueuil 6,328,979 B1 12/2001 Yamashita et al. (CA) 7,001,592 B1 2/2006 Traynor et al. 7,795,309 B2 9/2010 Kisak et al. (73) Assignee: Crescita Therapeutics Inc., 8,343,962 B2 1/2013 Kisak et al. Mississauga (CA) 8,513,304 B2 8, 2013 Kisak et al. 8,535,692 B2 9/2013 Pongpeerapat et al. (*) Notice: Subject to any disclaimer, the term of this 9,308,181 B2* 4/2016 Kisak ..................... A61K 47/12 patent is extended or adjusted under 35 2002fOOO6435 A1 1/2002 Samuels et al. 2002fOO64524 A1 5, 2002 Cevc U.S.C. 154(b) by 204 days. 2005, OO 14823 A1 1/2005 Soderlund et al. This patent is Subject to a terminal dis 2005.00754O7 A1 4/2005 Tamarkin et al. -

(12) Patent Application Publication (10) Pub. No.: US 2009/0005722 A1 Jennings-Spring (43) Pub
US 20090005722A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0005722 A1 Jennings-Spring (43) Pub. Date: Jan. 1, 2009 (54) SKIN-CONTACTING-ADHESIVE FREE Publication Classification DRESSING (51) Int. Cl. Inventor: Barbara Jennings-Spring, Jupiter, A61N L/30 (2006.01) (76) A6F I3/00 (2006.01) FL (US) A6IL I5/00 (2006.01) Correspondence Address: AOIG 7/06 (2006.01) Irving M. Fishman AOIG 7/04 (2006.01) c/o Cohen, Tauber, Spievack and Wagner (52) U.S. Cl. .................. 604/20: 602/43: 602/48; 4771.5; Suite 2400, 420 Lexington Avenue 47/13 New York, NY 10170 (US) (57) ABSTRACT (21) Appl. No.: 12/231,104 A dressing having a flexible sleeve shaped to accommodate a Substantially cylindrical body portion, the sleeve having a (22) Filed: Aug. 29, 2008 lining which is substantially non-adherent to the body part being bandaged and having a peripheral securement means Related U.S. Application Data which attaches two peripheral portions to each other without (63) Continuation-in-part of application No. 1 1/434,689, those portions being circumferentially adhered to the sleeve filed on May 16, 2006. portion. Patent Application Publication Jan. 1, 2009 Sheet 1 of 9 US 2009/0005722 A1 Patent Application Publication Jan. 1, 2009 Sheet 2 of 9 US 2009/0005722 A1 10 8 F.G. 5 Patent Application Publication Jan. 1, 2009 Sheet 3 of 9 US 2009/0005722 A1 13 FIG.6 2 - Y TIII Till "T fift 11 10 FIG.7 8 13 6 - 12 - Timir" "in "in "MINIII. -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9 -

Study of Purine Metabolism in a Xanthinuric Female
Study of Purine Metabolism in a Xanthinuric Female MICHAEL J. BRADFORD, IRWIN H. KRAKOFF, ROBERT LEEPER, and M. EARL BALis From the Sloan-Kettering Institute, Sloan-Kettering Division of Cornell University Graduate School of Medical Sciences, and the Department of Medicine of Memorial and James Ewing Hospitals, New York 10021 A B S TR A C T A case of xanthinuria is briefly de- Case report in brief. The patient is a 62 yr old scribed, and the results of in vivo studies with Puerto Rican grandmother, whose illness is de- 4C-labeled oxypurines are discussed. The data scribed in detail elsewhere.' Except for mild pso- demonstrate that the rate of the turnover of uric riasis present for 30 yr, she has been in good acid is normal, despite an extremely small uric health. On 26 June 1966, the patient was admitted acid pool. Xanthine and hypoxanthine pools were to the Second (Cornell) Medical Service, Bellevue measured and their metabolism evaluated. The Hospital, with a 3 day history of pain in the right bulk of the daily pool of 276 mg of xanthine, but foot and fever. The admission physical examina- only 6% of the 960 mg of hypoxanthine, is ex- tion confirmed the presence of monoarticular ar- creted. Thus, xanthine appears to be a metabolic thritis and mild psoriasis. The patient's course in end product, whereas hypoxanthine is an active the hospital was characterized by recurrent fevers intermediate. Biochemical implications of this find- to 104°F and migratory polyarthritis, affecting ing are discussed. both ankles, knees, elbows, wrists, and hands over a 6 wk period. -

Marrakesh Agreement Establishing the World Trade Organization
No. 31874 Multilateral Marrakesh Agreement establishing the World Trade Organ ization (with final act, annexes and protocol). Concluded at Marrakesh on 15 April 1994 Authentic texts: English, French and Spanish. Registered by the Director-General of the World Trade Organization, acting on behalf of the Parties, on 1 June 1995. Multilat ral Accord de Marrakech instituant l©Organisation mondiale du commerce (avec acte final, annexes et protocole). Conclu Marrakech le 15 avril 1994 Textes authentiques : anglais, français et espagnol. Enregistré par le Directeur général de l'Organisation mondiale du com merce, agissant au nom des Parties, le 1er juin 1995. Vol. 1867, 1-31874 4_________United Nations — Treaty Series • Nations Unies — Recueil des Traités 1995 Table of contents Table des matières Indice [Volume 1867] FINAL ACT EMBODYING THE RESULTS OF THE URUGUAY ROUND OF MULTILATERAL TRADE NEGOTIATIONS ACTE FINAL REPRENANT LES RESULTATS DES NEGOCIATIONS COMMERCIALES MULTILATERALES DU CYCLE D©URUGUAY ACTA FINAL EN QUE SE INCORPOR N LOS RESULTADOS DE LA RONDA URUGUAY DE NEGOCIACIONES COMERCIALES MULTILATERALES SIGNATURES - SIGNATURES - FIRMAS MINISTERIAL DECISIONS, DECLARATIONS AND UNDERSTANDING DECISIONS, DECLARATIONS ET MEMORANDUM D©ACCORD MINISTERIELS DECISIONES, DECLARACIONES Y ENTEND MIENTO MINISTERIALES MARRAKESH AGREEMENT ESTABLISHING THE WORLD TRADE ORGANIZATION ACCORD DE MARRAKECH INSTITUANT L©ORGANISATION MONDIALE DU COMMERCE ACUERDO DE MARRAKECH POR EL QUE SE ESTABLECE LA ORGANIZACI N MUND1AL DEL COMERCIO ANNEX 1 ANNEXE 1 ANEXO 1 ANNEX -

Complete Deficiency of Adenine Phosphoribosyltransferase
Arch Dis Child: first published as 10.1136/adc.54.1.25 on 1 January 1979. Downloaded from Archives of Disease in Childhood, 1979, 54, 25-31 Complete deficiency of adenine phosphoribosyltransferase A third case presenting as renal stones in a young child T. M. BARRATT, H. A. SIMMONDS, J. S. CAMERON, C. F. POTTER, G. A. ROSE, D. G. ARKELL, AND D. I. WILLIAMS Department of Medicine, Guy's Hospital, The Hospital for Sick Children, Great Ormond Street, and the Institute of Urology, St Philip's Hospital, London SUMMARY We report a third case of 2, 8-dihydroxyadenine stones in a child with a complete lack of the adenine salvage enzyme-adenine phosphoribosyltransferase (APRT). The propositus, a 20-month-old girl of consanguineous Arab parents, presented with multiple urinary tract infections and supposed 'uric acid' stones in the right renal pelvis and left ureter. Both parents and one brother were heterozygotes for the defect, in keeping with an autosomal recessive mode of inheritance. In contrast with the other purine salvage enzyme disorder of childhood with true uric acid stones (the Lesch-Nyhan syndrome), uric acid excretion was normal in all family members. As in our previous case, treatment with allopurinol, without alkali, has eliminated the urinary excretion of 2, 8-dihy- droxyadenine: the stones were removed surgically. 2, 8-Dihydroxyadenine should be considered in any child thought to have uric acid stones and tests made to distinguish the two compounds. Many urinary stones or crystals identified in children AMP IMP result from the overexcretion of normally minor http://adc.bmj.com/ urinary constituents, compounds of limited solubility whose overexcretion may be the direct consequence adenosine inosine of a block in an essential step in a metabolic pathway. -

Xanthine Urolithiasis: Inhibitors of Xanthine Crystallization
bioRxiv preprint doi: https://doi.org/10.1101/335364; this version posted May 31, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Xanthine urolithiasis: Inhibitors of xanthine crystallization Authors: Felix Grases, Antonia Costa-Bauza, Joan Roig, Adrian Rodriguez Laboratory of Renal Lithiasis Research, Faculty of Sciences, University Institute of Health Sciences Research (IUNICS-IdISBa), University of Balearic Islands, Ctra. de Valldemossa, km 7.5, 07122 Palma de Mallorca, Spain. Corresponding author: Felix Grases. Laboratory of Renal Lithiasis Research, Faculty of Sciences, University Institute of Health Sciences Research (IUNICS), University of Balearic Islands, Ctra. de Valldemossa, km 7.5, 07122 Palma de Mallorca, Spain. Telephone number: +34 971 17 32 57 e-mail: [email protected] Key words: xanthine lithiasis, 7-Methylxanthine, 3-Methylxanthine, Theobromine. 1 bioRxiv preprint doi: https://doi.org/10.1101/335364; this version posted May 31, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Abstract OBJECTIVE. To identify in vitro inhibitors of xanthine crystallization that have potential for inhibiting the formation of xanthine crystals in urine and preventing the development of the renal calculi in patients with xanthinuria. METHODS. The formation of xanthine crystals in synthetic urine and the effects of 10 potential crystallization inhibitors were assessed using a kinetic turbidimetric system with a photometer. -

Customs Tariff - Schedule
CUSTOMS TARIFF - SCHEDULE 99 - i Chapter 99 SPECIAL CLASSIFICATION PROVISIONS - COMMERCIAL Notes. 1. The provisions of this Chapter are not subject to the rule of specificity in General Interpretative Rule 3 (a). 2. Goods which may be classified under the provisions of Chapter 99, if also eligible for classification under the provisions of Chapter 98, shall be classified in Chapter 98. 3. Goods may be classified under a tariff item in this Chapter and be entitled to the Most-Favoured-Nation Tariff or a preferential tariff rate of customs duty under this Chapter that applies to those goods according to the tariff treatment applicable to their country of origin only after classification under a tariff item in Chapters 1 to 97 has been determined and the conditions of any Chapter 99 provision and any applicable regulations or orders in relation thereto have been met. 4. The words and expressions used in this Chapter have the same meaning as in Chapters 1 to 97. Issued January 1, 2019 99 - 1 CUSTOMS TARIFF - SCHEDULE Tariff Unit of MFN Applicable SS Description of Goods Item Meas. Tariff Preferential Tariffs 9901.00.00 Articles and materials for use in the manufacture or repair of the Free CCCT, LDCT, GPT, UST, following to be employed in commercial fishing or the commercial MT, MUST, CIAT, CT, harvesting of marine plants: CRT, IT, NT, SLT, PT, COLT, JT, PAT, HNT, Artificial bait; KRT, CEUT, UAT, CPTPT: Free Carapace measures; Cordage, fishing lines (including marlines), rope and twine, of a circumference not exceeding 38 mm; Devices for keeping nets open; Fish hooks; Fishing nets and netting; Jiggers; Line floats; Lobster traps; Lures; Marker buoys of any material excluding wood; Net floats; Scallop drag nets; Spat collectors and collector holders; Swivels.