Enoxaparin Prescribing Information Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Enoxaparin Sodium Solution for Injection, Manufacturer's Standard
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PrLOVENOX® Enoxaparin sodium solution for injection 30 mg in 0.3 mL solution (100 mg/mL), pre-filled syringes for subcutaneous or intravenous injection 40 mg in 0.4 mL solution (100 mg/mL), pre-filled syringes for subcutaneous or intravenous injection 60 mg in 0.6 mL solution (100 mg/mL), pre-filled syringes for subcutaneous or intravenous injection 80 mg in 0.8 mL solution (100 mg/mL), pre-filled syringes for subcutaneous or intravenous injection 100 mg in 1 mL solution (100 mg/mL), pre-filled syringes for subcutaneous or intravenous injection 300 mg in 3 mL solution (100 mg/mL), multidose vials for subcutaneous or intravenous injection PrLOVENOX® HP Enoxaparin sodium (High Potency) solution for injection 120 mg in 0.8 mL solution (150 mg/mL), pre-filled syringes for subcutaneous or intravenous injection 150 mg in 1 mL solution (150 mg/mL), pre-filled syringes for subcutaneous or intravenous injection Manufacturer’s standard Anticoagulant/Antithrombotic Agent ATC Code: B01AB05 Product Monograph – LOVENOX (enoxaparin) Page 1 of 113 sanofi-aventis Canada Inc. Date of Initial Approval: 2905 Place Louis-R.-Renaud February 9, 1993 Laval, Quebec H7V 0A3 Date of Revision September 7, 2021 Submission Control Number: 252514 s-a version 15.0 dated September 7, 2021 Product Monograph – LOVENOX (enoxaparin) Page 2 of 113 TABLE OF CONTENTS Sections or subsections that are not applicable at the time of authorization are not listed. TABLE OF CONTENTS .............................................................................................................. -

Rivaroxaban Versus Enoxaparin for Thromboprophylaxis After Hip
International Journal of Orthopaedics Sciences 2020; 6(3): 90-95 E-ISSN: 2395-1958 P-ISSN: 2706-6630 IJOS 2020; 6(3): 90-95 Rivaroxaban versus enoxaparin for © 2020 IJOS www.orthopaper.com thromboprophylaxis after hip arthroplasty Received: 20-05-2020 Accepted: 22-06-2020 Dr. Lenin Ligu and Dr. Moji Jini Dr. Lenin Ligu Assistant professor. Tomo Riba Institute of Health and Medical DOI: https://doi.org/10.22271/ortho.2020.v6.i3b.2183 Sciences (TRIHMS), Arunachal Pradesh, India Abstract Background: Introduction: Total hip arthroplasty (THA) is a successful surgical procedure for reducing Dr. Moji Jini pain and improving physical function in osteoarthritis. It is one of the most effective orthopaedic Director, Tomo Riba Institute of procedures. Health and Medical Sciences, Material and methods: Patients were eligible for the study if they were aged 18 years or older and were Arunachal Pradesh, India scheduled for total hip arthroplasty. Patients were ineligible if they were scheduled to undergo staged, bilateral hip arthroplasty were pregnant or breastfeeding, had active bleeding or a high risk of bleeding, or any disorder contraindicating the use of Rivaroxaban/enoxaparin that might necessitate enoxaparin dose adjustment. Results: Of the 52 patients enrolled in study, 52 patients were randomized to receive either Rivaroxaban (n=26) or enoxaparin (n=26). The baseline demographic characteristics of the two randomized treatment groups are well balanced as described in Table 1. The surgical characteristics are described in Table 2. Conclusion: Rivaroxaban, given as a once-daily 10 mg fixed dose 6–8 h postoperatively, is the first new oral anticoagulant to significantly reduce the incidence of venous thromboembolism after total knee arthroplasty, compared with enoxaparin 30 mg twice daily, starting 12–24 h postoperatively, without a significant difference in the risk of major or clinically relevant bleeding. -

Anticoagulation Dosing Guideline for Adult COVID-19 Patients
Anticoagulation Dosing Guideline for Adult COVID-19 Patients Enoxaparin is the preferred first line anticoagulant for patients diagnosed with COVID-19. The incidence of HIT with enoxaparin is less than 1%. VTE Prophylaxis: VTE prophylaxis will be considered for COVID-19 patients who are low risk. Low risk COVID-19 patient 1. Not receiving mechanical ventilation 2. D-Dimer < 6 mg/L 3. ESRD on iHD without clotting Kidney Function BMI (kg/m2) Dosing of Enoxaparin Concern for HIT or LMWH Failure CrCL ≥ 30 mL/min 18.5-39.9 30mg SUBQ Q12H Consult Hematology 40-49.9 40mg SUBQ Q12H ≥ 50 60mg SUBQ Q12H CrCL < 30 mL/min 18.5-39.9 30mg SUBQ Q24H Consult Hematology OR ≥ 40 40mg SUBQ Q24H ESRD/AKI on RRT Special Population: < 18.5 (or weight < 50kg) Heparin 2500 SUBQ Q8H Consult Hematology *Contraindications: Platelets < 25 K/uL or Fibrinogen < 50 mg/dL or active bleeding Therapeutic anticoagulation Therapeutic anticoagulation will be considered for COVID-19 patients who are considered high risk or diagnosed with an acute VTE. High risk COVID-19 patient (for all hospitalized patients): Receiving mechanical ventilation AND D-dimer > 6 mg/L OR Acute kidney injury (Scr increase 0.3 mg/dL above baseline) +/- CVVHD/AVVHD/SLED or IHD with clotting Anti-Xa level goals for enoxaparin therapy (when indicated): 1. Therapeutic peak LMWH level (Drawn 4 hours after 3rd dose): 0.6-1 anti-Xa units/mL 2. Therapeutic trough LMWH level (Drawn 1 hour prior to 3rd dose): < 0.5 anti-Xa units/mL Kidney Function BMI Dosing of Enoxaparin Concern for HIT or (kg/m2) LMWH -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Lovenox EMEA-H-A-30-1429
15 December 2016 EMA/134171/2017 Committee for Medicinal Products for Human Use Assessment report Referral under Article 30 of Directive 2001/83/EC Lovenox and associated names INN: enoxaparin Procedure number: EMEA/H/A-30/1429 Note: Assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted. 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2017. Reproduction is authorised provided the source is acknowledged. Table of contents Table of contents ......................................................................................... 2 1. Background information .......................................................................... 3 2. Scientific discussion ................................................................................ 3 2.1. Introduction ...................................................................................................... 3 2.2. Critical Evaluation .............................................................................................. 3 2.2.1. Product information ......................................................................................... 3 2.2.2. Risk Management Plan ................................................................................... 24 3. Recommendation .................................................................................. -

Protective Effect of Diclofenac and Enoxaparin in L- Asparaginase Induced Acute Pancreatitis in Rats
Advances in Environmental Biology, 10(9) September 2016, Pages: 30-41 AENSI Journals Advances in Environmental Biology ISSN-1995-0756 EISSN-1998-1066 Journal home page: http://www.aensiweb.com/AEB/ Protective Effect of Diclofenac and Enoxaparin in L- Asparaginase Induced Acute Pancreatitis in Rats 1Amr El-nashar, 2Amira M. Abo-Youssef, 3Ebtehal El-demerdash 1Clinical Research Coordinator, Hematology Malignancy, Children Cancer Hospital Egypt, CCHE 57357, 11411, 2Department of Pharmacology& Toxicology, Faculty of Pharmacy, Benisueif University, Benisueif, Egypt, 62514 3Department of Pharmacology & Toxicology, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt, 11566 Address For Correspondence: Amr El-nashar, Clinical Research Coordinator, Hematology Malignancy, Children Cancer Hospital Egypt, CCHE 57357, 11411, E-mail: [email protected] This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Received 12 July 2016; Accepted 18 September 2016; Available online 22 September 2016 ABSTRACT Objectives: This study was designed to evaluate the protective effect ofenoxaparinanddiclofenac againstL-asparaginase induced pancreatitis. Methods: Acute pancreatitis was induced in rats by intramuscular injection of L-asparaginase (1,000 I.U/Kg) given daily for five days. Enoxaparin was given subcutaneous (100 I.U/Kg) and diclofenac was given intraperitoneal (2 mg/Kg) daily for five days. Then, markers of pancreatic injury, lipids, immune cell infiltration and oxidative stress were analyzed with histopathological examination of the pancreatic tissue. Results: During acutepancreatitis, oxidative stress markerswere significantly changed as indicated by reduced tissue glutathione and increased malondialdehyde levels. This wasaccompaniedby a significant increase in immune cells infiltration as indicated by thehigh level of myeloperoxidase (MPO) andpro-inflammatory cytokine TNF-alpha. -

Low-Molecular-Weight Heparins in the Treatment of Acute Coronary Syndromes
REVIEW ARTICLE Low-Molecular-Weight Heparins in the Treatment of Acute Coronary Syndromes Alexander G. G. Turpie, MD; Elliott M. Antman, MD latelet aggregation and activation of coagulation are key events in the development of acute coronary syndromes. Patients with an acute coronary syndrome are at high risk of death or myocardial infarction, and hence there is a strong rationale for the use of antithrombotic agents. Heparin has been shown to reduce the risk of death or myocar- Pdial infarction in aspirin-treated patients with acute coronary syndromes, but it has a number of limitations, including the need for regular monitoring and the risk of hemorrhage and thrombo- cytopenia. Low-molecular-weight heparins offer a number of practical and clinical advantages over unfractionated heparin, such as higher bioavailability and administration by subcutaneous injec- tion. Several low-molecular-weight heparins are available that differ in their biochemical and phar- macologic properties, and it is not possible to predict their clinical efficacy from their pharmaco- logic profile. The decision regarding the use of a specific low-molecular-weight heparin should be based on the efficacy and safety data available for each product. In clinical trials comparing low- molecular-weight heparin with heparin, only enoxaparin sodium has been shown to reduce the risk of coronary events in patients with non–ST segment elevation acute coronary ischemia. Arch Intern Med. 2001;161:1484-1490 Clinical and pathologic studies have high- gina and non–Q wave MI indicates that the lighted the importance of plaque rupture culprit artery is only partially or intermit- and platelet aggregation in the pathogen- tently occluded or that a rich collateral cir- esis of the acute coronary syndromes (ACS) culation exists. -

Estonian Statistics on Medicines 2013 1/44
Estonian Statistics on Medicines 2013 DDD/1000/ ATC code ATC group / INN (rout of admin.) Quantity sold Unit DDD Unit day A ALIMENTARY TRACT AND METABOLISM 146,8152 A01 STOMATOLOGICAL PREPARATIONS 0,0760 A01A STOMATOLOGICAL PREPARATIONS 0,0760 A01AB Antiinfectives and antiseptics for local oral treatment 0,0760 A01AB09 Miconazole(O) 7139,2 g 0,2 g 0,0760 A01AB12 Hexetidine(O) 1541120 ml A01AB81 Neomycin+Benzocaine(C) 23900 pieces A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+Thymol(dental) 2639 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+Cetylpyridinium chloride(gingival) 179340 g A01AD81 Lidocaine+Cetrimide(O) 23565 g A01AD82 Choline salicylate(O) 824240 pieces A01AD83 Lidocaine+Chamomille extract(O) 317140 g A01AD86 Lidocaine+Eugenol(gingival) 1128 g A02 DRUGS FOR ACID RELATED DISORDERS 35,6598 A02A ANTACIDS 0,9596 Combinations and complexes of aluminium, calcium and A02AD 0,9596 magnesium compounds A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 591680 pieces 10 pieces 0,1261 A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 1998558 ml 50 ml 0,0852 A02AD82 Aluminium aminoacetate+Magnesium oxide(O) 463540 pieces 10 pieces 0,0988 A02AD83 Calcium carbonate+Magnesium carbonate(O) 3049560 pieces 10 pieces 0,6497 A02AF Antacids with antiflatulents Aluminium hydroxide+Magnesium A02AF80 1000790 ml hydroxide+Simeticone(O) DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 34,7001 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 3,5364 A02BA02 Ranitidine(O) 494352,3 g 0,3 g 3,5106 A02BA02 Ranitidine(P) -

Addition of Low Molecular Weight Heparin to Antiplatelet in Patients
urologic e al Soliman f N D i et al., J Neurol Disord 2019, 7:1 o s l o a r n d r e DOI: 10.4172/2329-6895.1000402 u r s o J Journal of Neurological Disorders ISSN: 2329-6895 Research Article Open Access Addition of Low Molecular Weight Heparin to Antiplatelet in Patients with Stroke Ineligible for Thrombolysis: Can it Improve Outcome? Hassan Ahmed Hashem Soliman1* , Yasser Hamed Mustafa1, Abdelhakeem Salem2 , Ahmed Yousry2 and Mohamed El-Refaey3 1Faculty of Medicine, Department of Neurology, Al-Azher University, Assuit Branch, Egypt 2Faculty of Medicine, Department of Cardiology, Zagazig University, Egypt 3Faculty of Medicine, Department of Radiology, Tanta University, Egypt Abstract Objective: Stroke is one of the most frequent causes of death and disability, urgent thrombolytic therapy can save thousands of lives and disabilities but unfortunately not all patients are eligible for thrombolysis. Dual antiplatelet are superior to anticoagulants in management of stroke and the use of full dose anticoagulants alone or in combination with aspirin is still debatable and carry the risk of hemorrhagic complications. Aim of study: We aimed to compare the use double antiplatelet (oral Aspirin plus Clopidogrel) versus oral aspirin plus subcutaneous low dose enoxaparin in treatment of progressing stroke ineligible for thrombolysis. Methods: The study was carried out on fifty-six patients presented with progressing stroke from February 2017 to the end of January 2019. Patients eligible for thrombolytic therapy (recombinant tissue plasminogen activator (r-TPA), TIA, Hemorrhagic stroke and stroke with stationary course were excluded from the study. The included patients were randomly divided into two groups, group (A) were treated with double antiplatelet aspirin 81 mg plus Clopidogrel 75 mg preceded by the loading dose of both and group (B) were treated with aspirin plus subcutaneous enoxaparin 40 mg (Clexane) once daily for 3 days. -

Reinvestigation of Synthesis of Enoxaparin Under PTC Conditions
Open Access Thrombosis & Haemostasis: Research Research Article Reinvestigation of Synthesis of Enoxaparin Under PTC Conditions Venkatanarayana P1, Prasanna B2* and Nareshvarma Seelam1 Abstract 1Department of Chemistry, Koneru Lakshmaiah Heparin is a mixture of Glycosaminoglycan (GAG) chains originating from Education Foundation, India porcine intestinal mucosa. It is used therapeutically as an anticoagulant for the 2Department of Chemistry, Chaitanya Post Graduate treatment and prevention of thrombosis. Glycosaminoglycans such as Heparin College (Autonomous), India (H) and Heparan Sulfate (HS) are considered attractive therapeutic agents *Corresponding author: Prasanna B, Department because they modulate many biological processes and have been implicated of Chemistry, Chaitanya Post Graduate College in numerous pathologies, including cardiovascular, cancer, inflammation, (Autonomous) Kishanpura, Hanamkonda, Warangal, metabolic, and neurodegenerative diseases and viral infections. These Telangana State 506 001. India biological functions are believed to be dependent on the interaction of these linear polysaccharides with key proteins such as growth factors, cytokines, Received: November 04, 2019; Accepted: December proteases, adhesion proteins, lipid binding proteins, etc., which have a heparin 05, 2019; Published: December 12, 2019 binding domain in common and are termed Heparin Binding Proteins (HBPs). The variability in unfractionated heparin’s pharmacokinetic properties and pharmacological effects led to the development of Low MW -
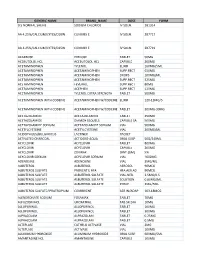
Generic Name Brand Name Dose Form 0.5 Normal Saline Sodium Chloride Iv Soln
GENERIC NAME BRAND_NAME DOSE FORM 0.5 NORMAL SALINE SODIUM CHLORIDE IV SOLN. 2B1314 AA 4.25%/CALCIUM/LYTES/D10W CLINIMIX E IV SOLN. 2B7717 AA 4.25%/CALCIUM/LYTES/D25W CLINIMIX E IV SOLN. 2B7719 ACARBOSE PRECOSE TABLET 50MG ACEBUTOLOL HCL ACEBUTOLOL HCL CAPSULE 200MG ACETAMINOPHEN TYLENOL ELIXIR 160MG/5ML ACETAMINOPHEN ACETAMINOPHEN SUPP.RECT 650MG ACETAMINOPHEN ACETAMINOPHEN DROPS 100MG/ML ACETAMINOPHEN ACETAMINOPHEN SUPP.RECT 325MG ACETAMINOPHEN FEVERALL SUPP.RECT 80MG ACETAMINOPHEN ACEPHEN SUPP.RECT 120MG ACETAMINOPHEN TYLENOL EXTRA STRENGTH TABLET 500MG ACETAMINOPHEN WITH CODEINE ACETAMINOPHEN W/CODEINE ELIXIR 120-12MG/5 ACETAMINOPHEN WITH CODEINE ACETAMINOPHEN W/CODEINE TABLET 300MG-30MG ACETAZOLAMIDE ACETAZOLAMIDE TABLET 250MG ACETAZOLAMIDE DIAMOX SEQUELS CAPSULE SA 500MG ACETAZOLAMIDE SODIUM ACETAZOLAMIDE SODIUM VIAL 500MG ACETYLCYSTEINE ACETYLCYSTEINE VIAL 200MG/ML ACIDOPHILUS/BULGARICUS LACTINEX PACKET ACTIVATED CHARCOAL ACTIDOSE-AQUA ORAL SUSP 50G/240ML ACYCLOVIR ACYCLOVIR TABLET 800MG ACYCLOVIR ACYCLOVIR CAPSULE 200MG ACYCLOVIR ZOVIRAX OINT.(GM) 5% ACYCLOVIR SODIUM ACYCLOVIR SODIUM VIAL 1000MG ADENOSINE ADENOSINE VIAL 3MG/ML ALBUTEROL ALBUTEROL AEROSOL 90MCG ALBUTEROL SULFATE PROVENTIL HFA HFA AER AD 90MCG ALBUTEROL SULFATE ALBUTEROL SULFATE VIAL-NEB. 2.5MG/0.5 ALBUTEROL SULFATE ALBUTEROL SULFATE SOLUTION 0.83MG/ML ALBUTEROL SULFATE ALBUTEROL SULFATE SYRUP 2MG/5ML ALBUTEROL SULFATE/IPRATROPIUM COMBIVENT AER W/ADAP 103-18MCG ALENDRONATE SODIUM FOSAMAX TABLET 70MG ALFUZOSIN HCL UROXATRAL TAB.SR 24H 10MG ALLOPURINOL ALLOPURINOL TABLET 100MG ALLOPURINOL ALLOPURINOL TABLET 300MG ALPRAZOLAM ALPRAZOLAM TABLET 0.25MG ALPRAZOLAM ALPRAZOLAM TABLET 0.5MG ALTEPLASE CATHFLO ACTIVASE VIAL 2MG ALTEPLASE ACTIVASE VIAL 100MG ALUMINUM HYDROXIDE ALUMINUM HYDROXIDE ORAL SUSP 600MG/5ML AMANTADINE HCL AMANTADINE CAPSULE 100MG GENERIC NAME BRAND_NAME DOSE FORM AMIKACIN SULFATE AMIKACIN SULFATE VIAL 250MG/ML AMILORIDE/HYDROCHLOROTHIAZIDE AMILORIDE HCL-HCTZ TABLET 5MG-50MG AMINO ACIDS 10% TRAVASOL IV SOLN. -

Acetylsalicylic Acid Compared with Enoxaparin for the Prevention of Thrombosis and Mechanical Ventilation in COVID‑19 Patients: a Retrospective Cohort Study
Clinical Drug Investigation (2021) 41:723–732 https://doi.org/10.1007/s40261-021-01061-2 ORIGINAL RESEARCH ARTICLE Acetylsalicylic Acid Compared with Enoxaparin for the Prevention of Thrombosis and Mechanical Ventilation in COVID‑19 Patients: A Retrospective Cohort Study Heba Wagih Abdelwahab1 · Shaker Wagih Shaltout2 · Hazem A. Sayed Ahmed3 · Ahmed Mahmoud Fouad4 · Eric Merrell5 · Jefrey B. Riley6 · Rasha Salama4 · Ahmed Gharib Abdelrahman3 · Edward Darling6 · Ghada Fadel11 · Mohamed S. A. Elfar7 · Khaled Sabry8 · Jafer Shah9 · Hossam Amin10 · Gary F. Nieman11 · Adel Mishriky4 · Hani Aiash3,6,11 Accepted: 6 July 2021 / Published online: 30 July 2021 © The Author(s), under exclusive licence to Springer Nature Switzerland AG 2021 Abstract Background and Objective Low-dose acetylsalicylic acid (ASA, aspirin) is a well-known and frequently studied drug for primary and secondary prevention of disease due to its anti-infammatory and coagulopathic efects. COVID-19 complications are attributed to the role of thrombo-infammation. Studies regarding the use of low-dose ASA in COVID-19 are limited. For this reason, we propose that the use of low-dose ASA may have protective efects in COVID-19–related thromboembolism and lung injury. This study was conducted to assess the efcacy of low-dose ASA compared with enoxaparin, an anticoagu- lant, for the prevention of thrombosis and mechanical ventilation. Methods We conducted a retrospective cohort study on COVID-19-confrmed hospitalized patients at the Mansoura Uni- versity Quarantine Hospital, outpatients, and home-isolated patients from September to December 2020 in Mansoura gov- ernorate, Egypt. Binary logistic regression analysis was used to assess the efect of ASA compared with enoxaparin on thromboembolism, and mechanical ventilation needs.