Pediatric Dermatology- Pigmented Lesions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

General Pathomorpholog.Pdf
Ukrаiniаn Medicаl Stomаtologicаl Аcаdemy THE DEPАRTАMENT OF PАTHOLOGICАL АNАTOMY WITH SECTIONSL COURSE MАNUАL for the foreign students GENERАL PАTHOMORPHOLOGY Poltаvа-2020 УДК:616-091(075.8) ББК:52.5я73 COMPILERS: PROFESSOR I. STАRCHENKO ASSOCIATIVE PROFESSOR O. PRYLUTSKYI АSSISTАNT A. ZADVORNOVA ASSISTANT D. NIKOLENKO Рекомендовано Вченою радою Української медичної стоматологічної академії як навчальний посібник для іноземних студентів – здобувачів вищої освіти ступеня магістра, які навчаються за спеціальністю 221 «Стоматологія» у закладах вищої освіти МОЗ України (протокол №8 від 11.03.2020р) Reviewers Romanuk A. - MD, Professor, Head of the Department of Pathological Anatomy, Sumy State University. Sitnikova V. - MD, Professor of Department of Normal and Pathological Clinical Anatomy Odessa National Medical University. Yeroshenko G. - MD, Professor, Department of Histology, Cytology and Embryology Ukrainian Medical Dental Academy. A teaching manual in English, developed at the Department of Pathological Anatomy with a section course UMSA by Professor Starchenko II, Associative Professor Prylutsky OK, Assistant Zadvornova AP, Assistant Nikolenko DE. The manual presents the content and basic questions of the topic, practical skills in sufficient volume for each class to be mastered by students, algorithms for describing macro- and micropreparations, situational tasks. The formulation of tests, their number and variable level of difficulty, sufficient volume for each topic allows to recommend them as preparation for students to take the licensed integrated exam "STEP-1". 2 Contents p. 1 Introduction to pathomorphology. Subject matter and tasks of 5 pathomorphology. Main stages of development of pathomorphology. Methods of pathanatomical diagnostics. Methods of pathomorphological research. 2 Morphological changes of cells as response to stressor and toxic damage 8 (parenchimatouse / intracellular dystrophies). -

Melanocytes and Their Diseases
Downloaded from http://perspectivesinmedicine.cshlp.org/ on October 2, 2021 - Published by Cold Spring Harbor Laboratory Press Melanocytes and Their Diseases Yuji Yamaguchi1 and Vincent J. Hearing2 1Medical, AbbVie GK, Mita, Tokyo 108-6302, Japan 2Laboratory of Cell Biology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892 Correspondence: [email protected] Human melanocytes are distributed not only in the epidermis and in hair follicles but also in mucosa, cochlea (ear), iris (eye), and mesencephalon (brain) among other tissues. Melano- cytes, which are derived from the neural crest, are unique in that they produce eu-/pheo- melanin pigments in unique membrane-bound organelles termed melanosomes, which can be divided into four stages depending on their degree of maturation. Pigmentation production is determined by three distinct elements: enzymes involved in melanin synthesis, proteins required for melanosome structure, and proteins required for their trafficking and distribution. Many genes are involved in regulating pigmentation at various levels, and mutations in many of them cause pigmentary disorders, which can be classified into three types: hyperpigmen- tation (including melasma), hypopigmentation (including oculocutaneous albinism [OCA]), and mixed hyper-/hypopigmentation (including dyschromatosis symmetrica hereditaria). We briefly review vitiligo as a representative of an acquired hypopigmentation disorder. igments that determine human skin colors somes can be divided into four stages depend- Pinclude melanin, hemoglobin (red), hemo- ing on their degree of maturation. Early mela- siderin (brown), carotene (yellow), and bilin nosomes, especially stage I melanosomes, are (yellow). Among those, melanins play key roles similar to lysosomes whereas late melanosomes in determining human skin (and hair) pigmen- contain a structured matrix and highly dense tation. -

Update on Challenging Disorders of Pigmentation in Skin of Color Heather Woolery-Lloyd, M.D
Update on Challenging Disorders of Pigmentation in Skin of Color Heather Woolery-Lloyd, M.D. Director of Ethnic Skin Care Voluntary Assistant Professor Miller/University of Miami School of Medicine Department of Dermatology and Cutaneous Surgery What Determines Skin Color? What Determines Skin Color? No significant difference in the number of melanocytes between the races 2000 epidermal melanocytes/mm2 on head and forearm 1000 epidermal melanocytes/mm2 on the rest of the body differences present at birth Jimbow K, Quevedo WC, Prota G, Fitzpatrick TB (1999) Biology of melanocytes. In I. M. Freedberg, A.Z. Eisen, K. Wolff,K.F. Austen, L.A. Goldsmith, S. I. Katz, T. B. Fitzpatrick (Eds.), Dermatology in General Medicine 5th ed., pp192-220, New York, NY: McGraw Hill Melanosomes in Black and White Skin Black White Szabo G, Gerald AB, Pathak MA, Fitzpatrick TB. Nature1969;222:1081-1082 Jimbow K, Quevedo WC, Prota G, Fitzpatrick TB (1999) Biology of melanocytes. In I. M. Freedberg, A.Z. Eisen, K. Wolff, K.F. Austen, L.A. Goldsmith, S. I. Katz, T. B. Fitzpatrick (Eds.), Dermatology in General Medicine 5th ed., pp192- 220, New York, NY: McGraw Hill Role of Melanin-Advantages Melanin absorbs and scatters energy from UV and visible light to protect epidermal cells from UV damage Disadvantages Inflammation or injury to the skin is almost immediately accompanied by alteration in pigmentation Hyperpigmentation Hypopigmentation Dyschromias Post-Inflammatory hyperpigmentation Acne Melasma Lichen Planus Pigmentosus Progressive Macular Hypomelanosis -

Dermatology DDX Deck, 2Nd Edition 65
63. Herpes simplex (cold sores, fever blisters) PREMALIGNANT AND MALIGNANT NON- 64. Varicella (chicken pox) MELANOMA SKIN TUMORS Dermatology DDX Deck, 2nd Edition 65. Herpes zoster (shingles) 126. Basal cell carcinoma 66. Hand, foot, and mouth disease 127. Actinic keratosis TOPICAL THERAPY 128. Squamous cell carcinoma 1. Basic principles of treatment FUNGAL INFECTIONS 129. Bowen disease 2. Topical corticosteroids 67. Candidiasis (moniliasis) 130. Leukoplakia 68. Candidal balanitis 131. Cutaneous T-cell lymphoma ECZEMA 69. Candidiasis (diaper dermatitis) 132. Paget disease of the breast 3. Acute eczematous inflammation 70. Candidiasis of large skin folds (candidal 133. Extramammary Paget disease 4. Rhus dermatitis (poison ivy, poison oak, intertrigo) 134. Cutaneous metastasis poison sumac) 71. Tinea versicolor 5. Subacute eczematous inflammation 72. Tinea of the nails NEVI AND MALIGNANT MELANOMA 6. Chronic eczematous inflammation 73. Angular cheilitis 135. Nevi, melanocytic nevi, moles 7. Lichen simplex chronicus 74. Cutaneous fungal infections (tinea) 136. Atypical mole syndrome (dysplastic nevus 8. Hand eczema 75. Tinea of the foot syndrome) 9. Asteatotic eczema 76. Tinea of the groin 137. Malignant melanoma, lentigo maligna 10. Chapped, fissured feet 77. Tinea of the body 138. Melanoma mimics 11. Allergic contact dermatitis 78. Tinea of the hand 139. Congenital melanocytic nevi 12. Irritant contact dermatitis 79. Tinea incognito 13. Fingertip eczema 80. Tinea of the scalp VASCULAR TUMORS AND MALFORMATIONS 14. Keratolysis exfoliativa 81. Tinea of the beard 140. Hemangiomas of infancy 15. Nummular eczema 141. Vascular malformations 16. Pompholyx EXANTHEMS AND DRUG REACTIONS 142. Cherry angioma 17. Prurigo nodularis 82. Non-specific viral rash 143. Angiokeratoma 18. Stasis dermatitis 83. -
A Case of Intradermal Melanocytic Nevus with Ossification (Nevus of Nanta)
197 A Case of Intradermal Melanocytic Nevus with Ossification (Nevus of Nanta) Young Bok Lee, M.D., Kyung Ho Lee, M.D., Chul Jong Park, M.D. Department of Dermatology, College of Medicine, The Catholic University of Korea, Seoul, Korea A 49-year-old woman presented with a 30-year history of asymptomatic plaque on her right temple. The histological examination revealed nests of nevus cells throughout the entire dermis. Bony spicules were seen just beneath the nevus cell nests in the lower dermis. Cutaneous ossification is an unusual event. Herein, we present a case of intradermal melanocytic nevus with unusual ossification (nevus of Nanta). To the best of our knowledge, this is the first such case report in the Korean literature. (Ann Dermatol (Seoul) 20(4) 197∼199, 2008) Key Words: Melanocytic nevus, Ossification INTRODUCTION drug intake or medical illness. The histological examination showed a dense proliferation of benign Ossification within the skin may occur in a nevus cells in the upper dermis. They were arranged variety of conditions, including pilomatricoma, basal in nests surrounding the hair follicles (Fig. 2). Bony cell carcinoma, appendageal and fibrous prolifera- spicules were seen in the lower dermis, underneath 1,2 tion, inflammation and trauma . The occurrence of the nevus cell nests. Some of them were compact ossification within a melanocytic nevus is an un- while others were surrounded by mature fatty tissue 3-5 usual event . (Fig. 3). Herein, we present a case of intradermal melano- cytic nevus with unusual ossification (nevus of Nanta). To the best our knowledge, this is the first such case report in the Korean literature. -

Short Course 11 Pigmented Lesions of the Skin
Rev Esp Patol 1999; Vol. 32, N~ 3: 447-453 © Prous Science, SA. © Sociedad Espafiola de Anatomfa Patol6gica Short Course 11 © Sociedad Espafiola de Citologia Pigmented lesions of the skin Chairperson F Contreras Spain Ca-chairpersons S McNutt USA and P McKee, USA. Problematic melanocytic nevi melanin pigment is often evident. Frequently, however, the lesion is solely intradermal when it may be confused with a fibrohistiocytic RH. McKee and F.R.C. Path tumor, particularly epithelloid cell fibrous histiocytoma (4). It is typi- cally composed of epitheliold nevus cells with abundant eosinophilic Brigham and Women’s Hospital, Harvard Medical School, Boston, cytoplasm and large, round, to oval vesicular nuclei containing pro- USA. minent eosinophilic nucleoli. Intranuclear cytoplasmic pseudoinclu- sions are common and mitotic figures are occasionally present. The nevus cells which are embedded in a dense, sclerotic connective tis- Whether the diagnosis of any particular nevus is problematic or not sue stroma, usually show maturation with depth. Less frequently the nevus is composed solely of spindle cells which may result in confu- depends upon a variety of factors, including the experience and enthusiasm of the pathologist, the nature of the specimen (shave vs. sion with atrophic fibrous histiocytoma. Desmoplastic nevus can be distinguished from epithelloid fibrous histiocytoma by its paucicellu- punch vs. excisional), the quality of the sections (and their staining), larity, absence of even a focal storiform growth pattern and SiQO pro- the hour of the day or day of the week in addition to the problems relating to the ever-increasing range of histological variants that we tein/HMB 45 expression. -

Megalencephaly and Macrocephaly
277 Megalencephaly and Macrocephaly KellenD.Winden,MD,PhD1 Christopher J. Yuskaitis, MD, PhD1 Annapurna Poduri, MD, MPH2 1 Department of Neurology, Boston Children’s Hospital, Boston, Address for correspondence Annapurna Poduri, Epilepsy Genetics Massachusetts Program, Division of Epilepsy and Clinical Electrophysiology, 2 Epilepsy Genetics Program, Division of Epilepsy and Clinical Department of Neurology, Fegan 9, Boston Children’s Hospital, 300 Electrophysiology, Department of Neurology, Boston Children’s Longwood Avenue, Boston, MA 02115 Hospital, Boston, Massachusetts (e-mail: [email protected]). Semin Neurol 2015;35:277–287. Abstract Megalencephaly is a developmental disorder characterized by brain overgrowth secondary to increased size and/or numbers of neurons and glia. These disorders can be divided into metabolic and developmental categories based on their molecular etiologies. Metabolic megalencephalies are mostly caused by genetic defects in cellular metabolism, whereas developmental megalencephalies have recently been shown to be caused by alterations in signaling pathways that regulate neuronal replication, growth, and migration. These disorders often lead to epilepsy, developmental disabilities, and Keywords behavioral problems; specific disorders have associations with overgrowth or abnor- ► megalencephaly malities in other tissues. The molecular underpinnings of many of these disorders are ► hemimegalencephaly now understood, providing insight into how dysregulation of critical pathways leads to ► -

Genes in Eyecare Geneseyedoc 3 W.M
Genes in Eyecare geneseyedoc 3 W.M. Lyle and T.D. Williams 15 Mar 04 This information has been gathered from several sources; however, the principal source is V. A. McKusick’s Mendelian Inheritance in Man on CD-ROM. Baltimore, Johns Hopkins University Press, 1998. Other sources include McKusick’s, Mendelian Inheritance in Man. Catalogs of Human Genes and Genetic Disorders. Baltimore. Johns Hopkins University Press 1998 (12th edition). http://www.ncbi.nlm.nih.gov/Omim See also S.P.Daiger, L.S. Sullivan, and B.J.F. Rossiter Ret Net http://www.sph.uth.tmc.edu/Retnet disease.htm/. Also E.I. Traboulsi’s, Genetic Diseases of the Eye, New York, Oxford University Press, 1998. And Genetics in Primary Eyecare and Clinical Medicine by M.R. Seashore and R.S.Wappner, Appleton and Lange 1996. M. Ridley’s book Genome published in 2000 by Perennial provides additional information. Ridley estimates that we have 60,000 to 80,000 genes. See also R.M. Henig’s book The Monk in the Garden: The Lost and Found Genius of Gregor Mendel, published by Houghton Mifflin in 2001 which tells about the Father of Genetics. The 3rd edition of F. H. Roy’s book Ocular Syndromes and Systemic Diseases published by Lippincott Williams & Wilkins in 2002 facilitates differential diagnosis. Additional information is provided in D. Pavan-Langston’s Manual of Ocular Diagnosis and Therapy (5th edition) published by Lippincott Williams & Wilkins in 2002. M.A. Foote wrote Basic Human Genetics for Medical Writers in the AMWA Journal 2002;17:7-17. A compilation such as this might suggest that one gene = one disease. -

Melasma on the Nape of the Neck in a Man
Letters to the Editor 181 Melasma on the Nape of the Neck in a Man Ann A. Lonsdale-Eccles and J. A. A. Langtry Sunderland Royal Hospital, Kayll Road, Sunderland SR4 7TP, UK. E-mail: [email protected] Accepted July 19, 2004. Sir, sunlight and photosensitizing agents may be more We report a 47-year-old man with light brown macular relevant. pigmentation on the nape of his neck (Fig. 1). It was The differential diagnosis for pigmentation at this site asymptomatic and had developed gradually over 2 years. includes Riehl’s melanosis, Berloque dermatitis and He worked outdoors as a pipe fitter on an oilrig module; poikiloderma of Civatte. Riehl’s melanosis typically however, he denied exposure at this site because he involves the face with a brownish-grey pigmentation; always wore a shirt with a collar that covered the biopsy might be expected to show interface change and affected area. However, on further questioning it liquefaction basal cell degeneration with a moderate transpired that he spent most of the day with his head lymphohistiocytic infiltrate, melanophages and pigmen- bent forward. This reproducibly exposed the area of tary incontinence in the upper dermis. It is usually pigmentation with a sharp cut off inferiorly at the level associated with cosmetic use and may be considered of his collar. He used various shampoos, aftershaves and synonymous with pigmented allergic contact dermatitis shower gels, but none was applied directly to that area. of the face (6, 7). Berloque dermatitis is considered to be His skin was otherwise normal and there was no family caused by a photoirritant reaction to bergapentin; it history of abnormal pigmentation. -
Diagnostic Pitfall of Localized Lentigo Accompanied by Post-Inflammatory
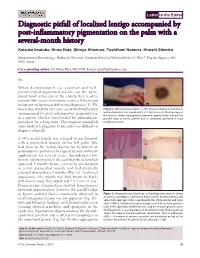
Letter to the Editor Diagnostic pitfall of localized lentigo accompanied by post-inflammatory pigmentation on the palm with a several-month history Keisuke Imafuku, Hiroo Hata, Shinya Kitamura, Toshifumi Nomura, Hiroshi Shimizu Department of Dermatology, Hokkaido University Graduate School of MedicineNorth 15, West 7, Kita-ku, Sapporo 060- 8638, Japan Corresponding author: Dr. Hiroo Hata, MD, PhD, E-mail: [email protected] Sir, When dermatologists see acquired and well- circumscribed pigmented macules on the palm, dorsal hand or forearm of the elderly, they tend to consider blue nevus, hematoma, nevus cell nevus and malignant melanoma as differential diagnoses [1]. We a b herein described the very rare case of localized lentigo Figure 1: Clinical manifestations. a. The macule is brown to dark black, accompanied by post-inflammatory pigmentation well-demarcated, flat, round and 3 x 4 mm in size. b. Dermoscopy of the macule shows homogenous brownish pigmentation without the in a patient who has been treated for palmoplanter parallel ridge or furrow pattern that is commonly observed in acral pustulosis for a long time. This eruption mimicked lentiginous lesion some kinds of lentiginous lesion and it was difficult to diagnose clinically. A 59-year-old female was referred to our hospital with a pigmented macule on her left palm. She had been to the family doctor for treatment of palmoplanter pustulosis by topical steroid ointment application for several years. According to the a history-taking interview, she said that the lesion had b appeared 5 months before referral to our hospital as a tiny pigmented macule and had gradually enlarged during those 5 months (Fig. -

Cutaneous Manifestations of Newborns in Omdurman Maternity Hospital
ﺑﺴﻢ اﷲ اﻟﺮﺣﻤﻦ اﻟﺮﺣﻴﻢ Cutaneous Manifestations of Newborns in Omdurman Maternity Hospital A thesis submitted in the partial fulfillment of the degree of clinical MD in pediatrics and child health University of Khartoum By DR. AMNA ABDEL KHALIG MOHAMED ATTAR MBBS University of Khartoum Supervisor PROF. SALAH AHMED IBRAHIM MD, FRCP, FRCPCH Department of Pediatrics and Child Health University of Khartoum University of Khartoum The Graduate College Medical and Health Studies Board 2008 Dedication I dedicate my study to the Department of Pediatrics University of Khartoum hoping to be a true addition to neonatal care practice in Sudan. i Acknowledgment I would like to express my gratitude to my supervisor Prof. Salah Ahmed Ibrahim, Professor of Peadiatric and Child Health, who encouraged me throughout the study and provided me with advice and support. I am also grateful to Dr. Osman Suleiman Al-Khalifa, the Dermatologist for his support at the start of the study. Special thanks to the staff at Omdurman Maternity Hospital for their support. I am also grateful to all mothers and newborns without their participation and cooperation this study could not be possible. Love and appreciation to my family for their support, drive and kindness. ii Table of contents Dedication i Acknowledgement ii Table of contents iii English Abstract vii Arabic abstract ix List of abbreviations xi List of tables xiii List of figures xiv Chapter One: Introduction & Literature Review 1.1 The skin of NB 1 1.2 Traumatic lesions 5 1.3 Desquamation 8 1.4 Lanugo hair 9 1.5 -

Cardiomyopathy Precision Panel Overview Indications
Cardiomyopathy Precision Panel Overview Cardiomyopathies are a group of conditions with a strong genetic background that structurally hinder the heart to pump out blood to the rest of the body due to weakness in the heart muscles. These diseases affect individuals of all ages and can lead to heart failure and sudden cardiac death. If there is a family history of cardiomyopathy it is strongly recommended to undergo genetic testing to be aware of the family risk, personal risk, and treatment options. Most types of cardiomyopathies are inherited in a dominant manner, which means that one altered copy of the gene is enough for the disease to present in an individual. The symptoms of cardiomyopathy are variable, and these diseases can present in different ways. There are 5 types of cardiomyopathies, the most common being hypertrophic cardiomyopathy: 1. Hypertrophic cardiomyopathy (HCM) 2. Dilated cardiomyopathy (DCM) 3. Restrictive cardiomyopathy (RCM) 4. Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) 5. Isolated Left Ventricular Non-Compaction Cardiomyopathy (LVNC). The Igenomix Cardiomyopathy Precision Panel serves as a diagnostic and tool ultimately leading to a better management and prognosis of the disease. It provides a comprehensive analysis of the genes involved in this disease using next-generation sequencing (NGS) to fully understand the spectrum of relevant genes. Indications The Igenomix Cardiomyopathy Precision Panel is indicated in those cases where there is a clinical suspicion of cardiomyopathy with or without the following manifestations: - Shortness of breath - Fatigue - Arrythmia (abnormal heart rhythm) - Family history of arrhythmia - Abnormal scans - Ventricular tachycardia - Ventricular fibrillation - Chest Pain - Dizziness - Sudden cardiac death in the family 1 Clinical Utility The clinical utility of this panel is: - The genetic and molecular diagnosis for an accurate clinical diagnosis of a patient with personal or family history of cardiomyopathy, channelopathy or sudden cardiac death.