The Chemistry of the Leather Industry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Care Label Recommendations
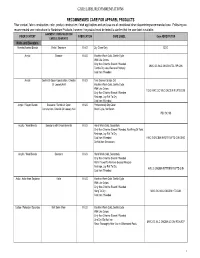
CARE LABEL RECOMMENDATIONS RECOMMENDED CARE FOR APPAREL PRODUCTS Fiber content, fabric construction, color, product construction, finish applications and end use are all considered when determining recommended care. Following are recommended care instructions for Nordstrom Products, however; the product must be tested to confirm that the care label is suitable. GARMENT/ CONSTRUCTION/ FIBER CONTENT FABRICATION CARE LABEL Care ABREVIATION EMBELLISHMENTS Knits and Sweaters Acetate/Acetate Blends Knits / Sweaters K & S Dry Clean Only DCO Acrylic Sweater K & S Machine Wash Cold, Gentle Cycle With Like Colors Only Non-Chlorine Bleach If Needed MWC GC WLC ONCBIN TDL RP CIIN Tumble Dry Low, Remove Promptly Cool Iron If Needed Acrylic Gentle Or Open Construction, Chenille K & S Turn Garment Inside Out Or Loosely Knit Machine Wash Cold, Gentle Cycle With Like Colors TGIO MWC GC WLC ONCBIN R LFTD CIIN Only Non-Chlorine Bleach If Needed Reshape, Lay Flat To Dry Cool Iron If Needed Acrylic / Rayon Blends Sweaters / Gentle Or Open K & S Professionally Dry Clean Construction, Chenille Or Loosely Knit Short Cycle, No Steam PDC SC NS Acrylic / Wool Blends Sweaters with Embelishments K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed, No Wring Or Twist Reshape, Lay Flat To Dry Cool Iron If Needed HWC S ONCBIN NWOT R LFTD CIIN DNID Do Not Iron Decoration Acrylic / Wool Blends Sweaters K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed Roll In Towel To Remove Excess Moisture Reshape, Lay Flat To Dry HWC S ONCBIN RITTREM -

Acacia Nilotica Pods)
J Biotechnol Biomed 2019; 2 (1): 015-023 DOI: 10.26502/jbb.2642-9128006 Research Article Innovation an Eco Friendly Technology: Tanning System using Semi Chrome and Improved Indigenous Tannins (Acacia Nilotica Pods) Haj Ali Alim A1*, Gasm elseed GA2, Ahmed AE3 1Department of National Leather Technology Center, Industrial Researcher and Consultant Center, Khartoum, Sudan 2Department of Chemical Engineering, University of Science and Technology, Omdurman, Sudan 3Department of Chemistry, Sudan University for Science and Technology, Khartoum, Sudan *Corresponding Author: Alim Abd Elgadir Haj Ali, Department of National Leather Technology Center, Industrial Researcher and Consultant Center, Khartoum, Sudan, P.O Box: 5008, E-mail: [email protected] Received: 08 January 2019; Accepted: 16 January 2019; Published: 21 January 2019 Abstract Semi-chrome tanned leathers were obtained using spray dried powder which were carried out using leaching of 70% crushed ‘Garad’ and 30% ‘Neem’ barks mixture to develop the fulfillment of ‘Garad’ tanning power. Tanning system was conducted in industrial research consultancies center, Sudan. Mechanical and physio-chemical analyses of the leather were executed using SLTC. Mechanical properties of the produced leather were compared with traditional tanned leather and the strengths, of tensile, one edge tear and two edges tear, of semi chrome tanned leather were: (200 kg/cm2, 52 and 100 kg/cm) respectively where the distension and strength of grain was (10 mm) and the thermal stability (100°C). The experimental explain that the blending ‘Garad-Neem’ significantly enhanced the quality of tannins powder and tanned leather. Keywords: Leather; Acacia Nilotica; Azadirachta Indica; Pre-tannage; Semi-Chrome Tannage; Mechanical; Physicochemical Properties 1. -

Silk, Linen, Leather, Denim, Grass, Cotton, Felt
Silk, linen, leather, denim, grass, cotton, felt. Natural materials keep the integrity of their shape yet hold an impression of the figure that has worn them. Fit-out for Olivia Spencer Bower speaks to the fabrics and forms that we live in. An assortment of garment patterns, building plans and the lifestyles of art heroines provide templates or underlying structures for Emma Fitts’ installation at the Ilam School of Fine Arts gallery. Adopting the layout of a modernist home and featuring a series of fabric hangings and clothing cutouts, Fitts’ work acutely relates to our situated knowledge, the proportions of the body and our experience of space, while also implying much less measurable qualities. Alternative histories that reveal the social relationships and values of a bygone era are incorporated to form a homage of sorts, though these references are evoked to shed light on the present–what it means to live and make work as an artist today. The gallery is physically divided by four large textile works, hung from the ceiling according to the walls shown in architectural plans for 15A Leinster Avenue, Christchurch–the former home of artist Olivia Spencer Bower (1905- 82). These soft walls enable visitors to occupy the space as though wandering through her actual house: living room, kitchen, sunroom, bedroom and studio. Designed in 1969 by architects Cowey and McGregor in the Christchurch style of neo-brutalism, the house was commissioned to accommodate the needs of a female artist living alone. There is an emphasis on form developed in relation to function and it is both refined and compact, manifesting these ideals. -

Position and Extent of the Elongation Zone in the Root Tip of the Broad Beanvicia Faba L
BIOLOGIA PLANTARUM (PRAHA) 8 (6) : 427--430, 1966 Position and Extent of the Elongation Zone in the Root Tip of the Broad Bean Vicia faba L. ALENA ADA_MKOVX and KAREL BENE~ Institute of Experimental Botany, Czechoslovak Academy of Sciences, Praha* Received November 9, 1965 Abstract. It was found by measuring the length of the cortex cells of the root tips of the broad bean Vieia faba L. that the beginning of the elongation zone lies at about 1--2 ram from the initials and its end at about 7--8 mm fl'om the initials. Shrinkage of the object during micro- technical treatment was negligible. The autonomy of the individual tissues of the root tip was taken into account. Introduetion Biochemical investigation of anatomically strictly defined parts of the root tip has become a common method for studying cell growth and tissue differ- antiation (BRow~r and BROADBENT 1951, I~OBINSO~r and BROW~r 1952). The results of the present work should serve as an anatomical basis for a series of papers dealing with the biochemical investigation of growth and differenti- ation processes in the bean root tip. The beginning and end of the elongation zone should be established here. Material and Methods Seeds of the broad bean Vicia faba L. cultivar Chlumeck~ were sterilized for 4 min with 70 % ethanol, washed for 8 h in running tap water and left to swell for 12 h in distilled water at 26 ~ C. The seeds were then placed onto wet paper wool laid on PVC plates with openings so that they lay above the opening. -

3) Renew the Colour of Your Suede Shoes with Waproo Suede and Nubuck Dye
Tel 0416 273 442 3) Renew the Colour of your Suede Shoes with Waproo Suede and Nubuck Dye Waproo Suede Nubuck dye is a spirit based dye that is used to efficiently colour or in other cases recolour suede as well as nubuck leather. The soft velvet like leather generally manufactured from the hide of cow is referred to as Suede. Nubuck is also a soft leather of durable quality. The difference between the two leathers is in the way they are prepared, the suede is sanded on the flesh split while nubuck is sanded on the outer surface. Waproo Suede and Nubuck dye is a permanent dye that is used to dye suede shoes, nubuck shoes, as well as other items made from such leather. Waproo Suede and Nubuck dye are packaged as a 50 ml bottle (except for black also packs in 500ml bottle) and comes in 4 different colours, black, brown, dark brown and navy blue. The Nubuck suede dye renews the colour of these items to make them look as good as when you just bought them. Items you will need: Waproo Suede and Nubuck Dye Waproo Suede and Nubuck Shampoo Waproo Suede and Nubuck Brush Follow the Instructions below to dye your suede and nubuck shoes: Insert the shoe tree or stuff your shoes with newspaper to prepare for the recolouring process. This will allow the wrinkles and other crevices to open and make a level surface to clean. Good preparation of the item to be dyed is the key to a satisfying result. If shoe lace is present, remove it first to clean the underneath. -

Winter 2019 Newsletter
Winter 2018 Aboriginal Education SHARING STORIES TO S UPPORT OUR CHILDREN, YOUTH, AND FAMILIES voice kiʔsuk kyukyit – Good afternoon, Ktunaxa territory xastsxlxalt – Good day, nslxcin from Sinixt territory Weyt-kp – Hello, Secwepemc territory Tawnshi - Hello, Michif (Language of the Metis People) Nestled in the mountains with still a light frosting of snow, Aboriginal Education is well underway for the 2018-2019 school year. We are moving forward with the Aboriginal Education department goals of improving literacy and numeracy through land based learning, providing opportunities for students to share their individual stories, and working toward Truth before Reconciliation, deepening relationships with the traditional territories our school district operates on and the Metis Nation. Created by the Aboriginal Education staff last year, we looked at the fire within and our daily work with students, families, and communities. This is such an exciting time in education, in history, and in our personal stories – as we all witness Indigenous ways of knowing blossom! What does blossoming look like in SD8? Whether that is through student pride in sharing a pow wow dance, a personal regalia story, a drum song, a new graphic design, entrance to College, or graduating with dignity and identity, or connecting to community teachings, or finding a sense of belonging in school, the faces of SD8 Aboriginal students, their learning, their successes and their dreams and goals are diverse. Yet, we see the goals of our District Enhancement Agreement flourishing through a variety of student successes, as individual as each of those bright faces. The District Aboriginal Education Advisory Committee and Elders’ Council, for the first time this year, welcomed guests from both the Syilx, Okanagan Nation Alliance, as well as guests from Enderby, from the Secwepemc Nation. -

Phase-Out of Chromium (III) in Leather Tanning
Phase-out of chromium (III) in leather tanning This case study aims to illustrate an alternative process. It is based on publicly available information on company's experience as well as on substance hazards, alternatives to the hazardous substance and regulatory information. The case study is neither complete nor comprehensive in illustrating all substitution options of a substance but rather exemplary. 1. Case description The conversion of hides or skin into leather involves a tanning process. In g e n e ra l , d e p e n d i n g u p o n the end application of leather, two tanning methods are used: vegetable tanning or chrome tanning. 80 – 90 % of tanneries around the world today use salts of trivalent chromium for tanning. The main chromium compound used for leather tanning is chromium (III) hydroxide sulphate, Cr(OH)SO4 (CAS No. 12336-95-7; EC No. 235-595-8). Chromium is associated with several negative effects for the environment and human health. The main hazards related to chromium (III) is that it can be oxidised to chromium (VI), e.g. at very low pH values when oxygen is present. Cr (VI) is a suspected carcinogen and causes skin sensitisation. Since chromium (III) can transform into chromium (VI), its use in articles that may be in direct skin contact, should be avoided. 1.1. Hazards of chromium (VI) Chromium (VI) causes an allergic contact dermatitis and sensitisation at very low concentrations. The risk assessment made by Denmark (also proposing a restriction to market certain articles of leather coming into direct contact with the skin) demonstrates that chromium (VI) present in shoes and other leather articles poses risks for consumers. -

Thesis-1949-S649l.Pdf (10.18Mb)
1 LEATHEBS EMPLOYED I ·l THE 'l'EAC I.NG OF LEA'll:iERCRAFT ii LEA'IDERS ff LOYED I THE 'l'E ' C I TG OF LEATHERCRAFT By HARRY LEE ..s -, ITH Bachelor of Science ort.h Texas State Teachers College Denton, Texas 1947 Submitted to the Department of Industrial Arts Education Oklahoma Agx-icultural and echenieal. College In Partial Fulfillment. of the Requirement.a For the Degree of TER OF SCIEJ'"CE 1949 iii 1iies1s .vis · r a · Head, School of Iniust.ria.l Arts Ed.ucation WX1 Engineering Shopwork 236 592 iv AC K.J.~O' LE.DGMEN'rs 'lhe writer expresses hi sincere appreciation to Dr. De itt Hunt,, Head of the Department.. of Iroustrial. Arts Fiiucation and Engineering Shopwork, Ok1ahoma Agricul.tural am · echanical College1 for his helpful assistance and guidance during the preparation and completion of thia study. Appreciation is extentled to nw wife, Eunice Blood mith, for her encouragement and help throughout the preparation of this study. V TABLE OF CONTENTS PAGE I. PRELIMI STATEMENTS ••••••••• • •• • l Purpose oft.he Study ••••••••••••• l '.Ille Importance of the Study ••••••••• 2 Delimit.ations • •. • • • • • • • • • • • • • • 2 Def'inition of Terms ............. 3 A Preview of Organization •••••••••• 4 II. HISTORICAL STUDY OF LET.HER • • •• • • •• • • 5 The Raw Material • • • • • ., • • • • • • • • • 5 'Ihe Evolution of Leather ••••••••••• 6 '!he Egyptian Leather • • • • • • • • • • • • • 7 Early .Arabian Leather • • • • • • • • • ••• 7 The Jewish Babylonian Leather •••••••• 8 Gl"ec ian Leatber • • • • • • • • • • • • • • • 8 Roman Leather • • • • • • • • • • • • • • • • 9 European Leather of the Middle Ages •••• • 9 Leather of the Far East • • • • • • • • • • • ll American Leather ••••••••••••••• 11 III. PRI CIPAL TYPES OF LEATHERCRAFT TERIALS • • • 14 Alligator • • • • • • • • • • • • • ••• .•• 14 Cabretta • • • • • • • • • • . -

Leather Care & Cleaning
L eather Care & Cleaning Guidelines for Cleaning Leather. Leather Care and Cleaning Guide. The Leather Institute. 2011 For more information or questions or comments please contact The Leather Institute at [email protected] General Leather Care and Cleaning Instructions A word about leather care products… There are many products available for cleaning leather and removing stains. The products best suited for cleaning leather are those recommended by the Leather Supplier. Townsend Leather recommends using ONLY The Leather Institute care and cleaning products. The Leather Institute products are especially formulated to work perfectly with Townsend Leather. When cleaning leather... Never use: Any High pH cleaner Cleaners that contain abrasives Cleaners that contain alcohol Cleaners that contain Butyl Cellosolve Any strong solvent Saddle soap Mink oil Wax Furniture polish Glass cleaner Or any strong solvents, abrasives or caustic household cleaners such as soap or dish detergent. These vary widely in strength and in compatibility with today's water-based leather finishes and may cause cracking or other damage to the leather surface. Direct sources of heat and extended exposure to heat sources and to direct sunlight may also be harmful to the leather and should be avoided. Always use: Leather care products recommended by The Leather Institute and only use those products in the prescribed method. The only acceptable products for use on Townsend Leather products are The Leather Institute Finished Leather Care Products or leather care products approved by Townsend Leather: Finished Leather Cleaner Finished Leather Cleaning Wipes Cleaner, Conditioner, Protector Ink & Stain Remover Stick 100% Cotton Terry Cloth Rags (White Only); commercially available A solution of distilled water a mild non-detergent soap Basic Leather Cleaning Techniques The most basic task at any level of cleaning is a thorough vacuuming. -

Analysis of Artificial Leather with Textile Fabric on the Backside
Volume 6, Issue 2, Fall2009 ANALYSIS OF ARTIFICIAL LEATHER WITH TEXTILE FABRIC ON THE BACKSIDE Darko Ujević, Stana Kovačević, *Larry C. Wadsworth, Ivana Schwarz, and Blaženka Brlobašić Šajatović University of Zagreb, Faculty of Textile Technology, Zagreb, Croatia *The University of Tennessee, Department of Materials Science and Engineering ABSTRACT The fundamental characteristics of a textile fabric intended for the vehicle interior is presented. Chemical and physical-mechanical properties of artificial leather with bonded textile fabric on the back side are analyzed. The most important parameters for leather durability are: breaking force and elongation-at-break, and these properties will be tested in different circular directions. Likewise, chemical properties of artificial leather and basic construction parameters of the textile fabric are investigated. When using artificial leather, physical-mechanical properties of artificial leather as well as the quality of the seams are most important. In addition to the results obtained, physical-mechanical properties and aesthetic evaluation of the joined places will be compared. Keywords: Artificial leather, Textile fabric on the back side, Joined place strength, Seam strength, Physical-mechanical properties Introduction pleather (plastic leather) is a slang term for synthetic leather made out of plastic, a In addition to ergonomically designed car portmanteau of plastic and leather, the term seats for keeping the body in a correct sitting can be either descriptive, or derogatory, position, it is important that the passenger depending upon the user (the derogatory use feels no bodily fatigue due to sitting implies use as a substitute for genuine discomfort. Pleasant contact between the animal hide leather to cut costs). -

Mangrove Arboretum
Mangrove arboretum The arboretum in Bac Lieu is a collection of mangrove species from the south of Viet Nam. The arboretum is a show case of species richness and thus contribute to the preservation and research of mangrove forests. Furthermore, it serves eco-tourism and is considered as learning resource on mangroves in the Mekong area for students and practitioners. Worldwide, about 73 different species are accounted as ‘true mangroves’. In a total, there are 39 true mangroves described for Vietnam and at least 27 can be found in the South of Vietnam. There are not many stands of natural old mangroves remaining in the Mekong Delta, most of the forest is reforested. For reforestation efforts in the past only few species were selected and the more important it is for the future to diversify the planting schemes since every species is the result of a long evolutionary adaptation process and thus much more resilient if planted at the right place with certain environmental conditions. Mangroves store about 78,8 tons of carbon per hectare and provide high ecological services such as being nursery and foraging space for numerous coastal shrimp, crab and fish species important to local fisheries and aquaculture. Pop-up content of CPMD layer Forests -> Arboretum: Bạc Liêu Mangrove Arboretum Area 5 ha Location Giong Nhan hamlet, Hiep Thanh commune, Bac Lieu town (district), Bac Lieu province No. Vietnamese Scientific name Use name 1 Bần ổi Sonneratia Wood is not good, lightweight, soft. It plays the role ovate Back of breaking wind, protecting the coastal area 2 Cóc đỏ Lumnitzera Wood is used in household appliances, for fuel. -

About Leather Types of Leather
About Leather Types of Leather Leather is an ancient, durable material created through a process of tanning animal rawhide to preserve it and make it pliable when dry. Many features of natural leather make it superior to synthetic products including durability, comfort, beauty, suppleness, and resilience. Plus, leather’s ability to patina and absorb body oils continues to enhance the leather’s appearance and makes it more beautiful over time. Leather can broadly be divided into full grain, enhanced grain, corrected grain, top grain and split leathers. Full Grain Leather Full grain leather has no surface alterations. The hide’s natural pores and grain textures are intact and it will develop a patina over time. The tiny pinholes dotting the surface indicate the hide’s open hair follicles. The presence of these hair follicles demonstrates a high quality surface which has not been altered to conceal flaws. This full grain surface breathes. It keeps the user Full grain comfortable as it adjusts to body temperature. Full grain leather is the highest quality, most beautiful, and most comfortable leather available. Enhanced Grain Leather Enhanced grain leather is a full grain with an artificial grain embossed over the natural grain. Enhanced grain leather has the same comfort and breathability of a full grain, but the surface has received minor alteration to improve grain Enhanced grain appearance. Corrected Grain Leather Corrected grain leather is produced from the upper portion of the hide. The surface is lightly sanded or refined then embossed with an artificial grain texture. Corrected grain leathers have a more consistent appearance across the entire surface.