Basal Salt Composition, Cytokinins, and Phenolic Binding Agents Influence in Vitro Growth and Ex Vitro Establishment of Magnolia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

THE Magnoliaceae Liriodendron L. Magnolia L
THE Magnoliaceae Liriodendron L. Magnolia L. VEGETATIVE KEY TO SPECIES IN CULTIVATION Jan De Langhe (1 October 2014 - 28 May 2015) Vegetative identification key. Introduction: This key is based on vegetative characteristics, and therefore also of use when flowers and fruits are absent. - Use a 10× hand lens to evaluate stipular scars, buds and pubescence in general. - Look at the entire plant. Young specimens, shade, and strong shoots give an atypical view. - Beware of hybridisation, especially with plants raised from seed other than wild origin. Taxa treated in this key: see page 10. Questionable/frequently misapplied names: see page 10. Names referred to synonymy: see page 11. References: - JDL herbarium - living specimens, in various arboreta, botanic gardens and collections - literature: De Meyere, D. - (2001) - Enkele notities omtrent Liriodendron tulipifera, L. chinense en hun hybriden in BDB, p.23-40. Hunt, D. - (1998) - Magnolias and their allies, 304p. Bean, W.J. - (1981) - Magnolia in Trees and Shrubs hardy in the British Isles VOL.2, p.641-675. - or online edition Clarke, D.L. - (1988) - Magnolia in Trees and Shrubs hardy in the British Isles supplement, p.318-332. Grimshaw, J. & Bayton, R. - (2009) - Magnolia in New Trees, p.473-506. RHS - (2014) - Magnolia in The Hillier Manual of Trees & Shrubs, p.206-215. Liu, Y.-H., Zeng, Q.-W., Zhou, R.-Z. & Xing, F.-W. - (2004) - Magnolias of China, 391p. Krüssmann, G. - (1977) - Magnolia in Handbuch der Laubgehölze, VOL.3, p.275-288. Meyer, F.G. - (1977) - Magnoliaceae in Flora of North America, VOL.3: online edition Rehder, A. - (1940) - Magnoliaceae in Manual of cultivated trees and shrubs hardy in North America, p.246-253. -

The Red List of Magnoliaceae Revised and Extended
The Red List of Magnoliaceae revised and extended Malin Rivers, Emily Beech, Lydia Murphy & Sara Oldfield BOTANIC GARDENS CONSERVATION INTERNATIONAL (BGCI) is a membership organization linking botanic gardens in over 100 countries in a shared commitment to biodiversity conservation, sustainable use and environmental education. BGCI aims to mobilize botanic gardens and work with partners to secure plant diversity for the Published by Botanic Gardens Conservation International Descanso House, 199 Kew Road, well-being of people and the planet. BGCI provides the Secretariat for Richmond, Surrey, TW9 3BW, UK. the IUCN/SSC Global Tree Specialist Group. © 2016 Botanic Gardens Conservation International ISBN-10: 1-905164-64-5 ISBN-13: 978-1-905164-64-6 Reproduction of any part of the publication for educational, conservation and other non-profit FAUNA & FLORA INTERNATIONAL (FFI) , founded in 1903 and the purposes is authorized without prior permission from world’s oldest international conservation organization, acts to conserve the copyright holder, provided that the source is fully acknowledged. threatened species and ecosystems worldwide, choosing solutions that are sustainable, are based on sound science and take account of Reproduction for resale or other commercial purposes human needs. is prohibited without prior written permission from the copyright holder. Recommended citation: Rivers, M., Beech, E., Murphy, L. and Oldfield, S. (2016). The Red List of Magnoliaceae - revised and extended. BGCI. Richmond, UK. AUTHORS Malin Rivers is the Red List Manager at BGCI. THE GLOBAL TREES CAMPAIGN (GTC) is undertaken through a Emily Beech is a Conservation Assistant at BGCI. partnership between BGCI and FFI. GTC’s mission is to prevent all tree Lydia Murphy is the Global Trees Campaign Intern species extinctions in the wild, ensuring their benefits for people, wildlife at BGCI. -

Trees K2 3/2/10 14:10 Page 1
Trees K Blad PGc 2.02.10:Trees K2 3/2/10 14:10 Page 1 Contents INTRODUCTION The Hemlocks 128 Figs and Mulberries 248 by Hugh Johnson The True Cedars 130 Beeches 250 The Larches 134 Southern Beeches 256 THE WORLD OF TREES The Plum Yews and Podocarps 138 Oaks 258 How a Tree Grows 10 The True Cypresses 140 Oaks of Europe and Asia 260 How a Tree Works 14 The False Cypresses 142 Oaks of North America 264 The Leaves 18 The Junipers 146 The Chestnuts 270 The Flowers 22 The Incense Cedars 150 The Birches 272 The Fruit 26 The Thujas 152 Alders 278 History in a Tree 28 The Yews 154 Hornbeams and Hazels 282 Roots and the Soil 30 The Sequoias 156 Walnuts 286 How Trees are Named 32 Asian Cypresses 160 Hickories and Wingnuts 288 Trees and the Weather 34 The Dwarf Conifers 162 Limes and Lindens or Basswoods 292 Zones of Hardiness 36 The Palms 166 Horse Chestnuts and Buckeyes 296 The Advance of Spring 38 Maples of North America 300 Forestry 40 Maples of the East 306 Trees Throughout History 44 Broadleaves Maples of Europe 310 Collectors and Creators 64 The Tulip Tree and Magnolias 174 Cashews and Sumachs 316 Propagation 70 The Bays and Laurels 180 Trees of Heaven and Cedrelas 318 Choosing the Species 72 The Eucalypts 186 Citrus Trees 320 Planning for Planting 76 The Willows 192 Dogwood, Davidia and Nyssa 322 Trees for Shade, Shelter and Seclusion 80 The Poplars, Cottonwoods and Aspens 196 Dove Trees and Black Gums 326 Planting Trees 82 Antipodeans 200 The Tea Family 328 Maintenance 86 The Legumes 204 Persimmons, Silverbells and Snowbells 332 Pruning and -

Issue 67 03-15 Studies on Magnolia
ISSUE 67 MACNOUA Studies On Magnolia Delavayi and Its Natural Forms Sun Weibang, Kong Fancai, and Luo GUIyen Background Franchet named Magnolia delavayi in 1889 based on the specimen collected in China in 1886 by the French missionary, Pere Jean M. Delavay. It was first introduced into cultivation in England by E. H. Wilson, who collected seeds during the autumn of 1899 from south- ern Yunnan. Since then, M delavayi has gradually spread imo many countries as an evergreen ornamental shrub. Magnolsa delavayi is a special species in Magnoliaceae. Its flower tepals open mostly in the evening and last only a few hours. In China, it is called the Holy Flower of Chinese Buddhism, or, in Chi- nese, Youtan Hua, which means "Flower Briefly as the Broad-leaved " Epiphyllum. (Editor's Note: Epiphyllum oxypetalum is a species of Orchid Cactus. There are about 20 spp. , all of which are native to the New World Tropics. The flowers are stunning but only bloom briefly at night. ) Before it was named, the Holy Flower of Chinese Bud- dhism had been cultivated in China as an ornamental, religious, and medicinal plant for over 800 years. Thus, most of the very old trees are commonly found near temples or in villages in Yunnan and other parts of China. For example: ~ An 800-year old M. delavayi grows in the yard of the Caoxi Temple at Arming county near Kunming in Yunnan. This tree is gm high, with a 74cm base-trunk diameter and still flowers well every year. ~ A 230-year old M. delavayi thrives in the Yufen Temple of Lijiang in the province of Yunnan. -

Phylogenetic Relationships in Magnoliaceae Subfam
Plant Syst. Evol. 242: 33–47 (2003) DOI 10.1007/s00606-003-0055-5 Phylogenetic relationships in Magnoliaceae subfam. Magnolioideae: a morphological cladistic analysis J. Li1 and J. G. Conran2 1Kunming Division of Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Kunming, Yunnan, P.R. China 2Centre for Evolutionary Biology and Biodiversity, Environmental Biology, School of Earth and Environmental Sciences, The University of Adelaide, South Australia Received December 13, 2001; accepted February 19, 2003 Published online: November 26, 2003 Ó Springer-Verlag 2003 Abstract. Relationships within Magnolioideae morphological data are needed to improve phylo- have been the subject of persistent debate; the genetic signal. Our results support the molecular main point at issue mostly being the disposition of analyses in suggesting that Magnolia is best con- tribes, genera and sections. A morphological cla- sidered to be a large and diverse genus, but that the distic analysis of the subfamily using Liriodendron relationships between the taxa within it require as the out-group showed that Magnolioideae con- more detailed clarification, with more extensive sisted of a large basal polytomy, but with five sampling and a combined molecular and morpho- resolved and variously supported clades. Manglie- logical approach being needed. tia constituted a clade with sect. Rytidospermum of Magnolia subg. Magnolia. Kmeria and Woonyoun- Key words: Angiosperm, Magnoliaceae, Magno- gia formed a pair. Pachylarnax, Parakmeria and lioideae, Phylogeny, morphology, relationships, Manglietiastrum were grouped together, and sect. Magnolia. Splendentes and Dugandiodendron also formed a pair. The largest and best supported clade consisted Introduction of Magnolia subg. Magnolia sects. Oyama and Maingola, Magnolia subg. -

Of Magnolia Delavayi
A brief report on micropropagation of a rare ornamental shrub —the red form of Magnolia delavayi Luo Gui-Fen aud Suu Wei-Bang Some years ago the red flowered form of Magnolia delavayi Franch. was found in the Hua-Fu mountains of Mou-Din County in Yunnan province of China. At that time, only one plant natura}ly grew among the mixed forest of Magnolia delavayi and Manglieiia invignie. It is said that it is a natural hybrid between these species, but so far we have no more evidence. 'Ihe Kumming Institute of Botany (Academia Sinica, Kumming. Yunnan, Chins} has carried out its introduction and cultivation ffom seed, and good results have been achieved although the progeny shows a very wide range of variation in flower color. Last year we started a project of vegetative propagation of the selected red form, including a study of micropropagation methods. So far there is no International report on the mtcropropagatton of the red form of Magnolia delavayi. and we are here reporting our preliminary results to TTNS members. Materials snd Methods: The explants tunx} were terminal buds which were cut ffom the vigomus plant inside the Institute in early spry. The explants were washed with dean water and the surface was cleaned by using 7N6 alcohoL Explants were then soaked in a 0. 196 solution of mercuric chloride for 5 minutes. 'Ihe steri}ized explants were again washed ffve to ten times in distilled water. The explants were placed on the media of Murashige gt Skoog (MS) and Vacin gr. Went (VW) with different combinations of benzyladenine (BA}, 0.50 — 5.00mg/L. -

Comparative Floral Anatomy and Ontogeny in Magnoliaceae
Pl. Syst. Evol. 258: 1–15 (2006) DOI 10.1007/s00606-005-0361-1 Comparative floral anatomy and ontogeny in Magnoliaceae F. Xu1 and P. J. Rudall2 1South China Botanical Garden, Academia Sinica, Guangzhou, China 2Royal Botanic Gardens, Kew, Richmond, Surrey, UK Received November 16, 2004; accepted June 9, 2005 Published online: March 8, 2006 Ó Springer-Verlag 2006 Abstract. Floral anatomy and ontogeny are de- Key words: Floral development, Floral morphology, scribed in six species of Magnoliaceae, representing Liriodendron, Magnolia, Michelia. the two subfamilies Liriodendroideae (Liriodendron chinese and L. tulipifera) and Magnolioideae, including species with terminal flowers (Magnolia Introduction championi, M. delavayi, M. grandiflora, M. pae- netalauma) and axillary flowers (Michelia crassipes). Magnoliaceae are a well-defined and horticul- The sequence of initiation of floral organs is from turally important family of about 230 species proximal to distal. The three distinct outermost of trees and shrubs characterised by large organs are initiated in sequence, but ultimately form flowers with numerous tepals and fertile parts a single whorl; thus their ontogeny is consistent with inserted separately on an elongated axis. More a tepal interpretation. Tepals are initiated in whorls, than 80% of species of Magnoliaceae are and the stamens and carpels are spirally arranged, though the androecium shows some intermediacy distributed in subtropical and tropical regions between a spiral and whorled arrangement. Carpels of eastern Asia; the remainder occur in Amer- are entirely free from each other both at primordial ica, indicating a relictual tropical disjunction stages and maturity. Ventral closure of the style (Azuma et al. 2001). Renewed debate on the ranges from open in Magnolia species examined to systematics of the family has been stimulated partially closed in Michelia crassipes and completely by several recent cladistic analyses, both closed in Liriodendron, resulting in a reduced stigma morphological (Li and Conran 2003) and surface. -

Pollen Morphology and Ultrastructure of Selected Species of Magnoliaceae
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by The University of North Carolina at Greensboro Pollen morphology and ultrastructure of selected species of Magnoliaceae By: Feng-Xia Xu a,*, Bruce K. Kirchoff b Xu, Feng-Xia, and B. K. Kirchoff. 2008. Pollen morphology and ultrastructure of selected species of Magnoliaceae. Review of Palaeobotany and Palynology 150: 140-153 Made available courtesy of ELSEVIER: http://www.elsevier.com/wps/find/journaldescription.cws_home/503359/description#description ***Note: Figures may be missing from this format of the document Abstract: The pollen morphology and ultrastructure of 20 species, representing eight genera of the Magnoliaceae are described based on observations with light, scanning and transmission electron microscopy. The family represents a homogeneous group from a pollen morphological point of view. The pollen grains are boat-shaped with a single elongate aperture on the distal face. The tectum is usually microperforate, rarely slightly or coarsely rugulose. Columellae are often irregular, but well-developed columellae do occur in some taxa. The endexine is distinct in 14 species, but difficult to discern in the genera Parakmeria, Kmeria and Tsoongiodendron. Within the aperture zone the exine elements are reduced to a thin foot layer. The intine has three layers with many vesicular-fibrillar components and tubular extensions in intine 1. The symmetry of the pollen grains, shape, type of aperture and ultrastructure of the intine show a remarkable uniformity in the family. Nevertheless there is variety in pollen size, ornamentation and the ultrastructure of the exine. The pollen of Magnoliaceae is an example of an early trend of specialization, and supports the view that Magnoliaceae are not one of the earliest lines in the phylogeny of flowering plants. -

Some Botanical Highlights in the Gardens in July 2017
Some botanical highlights in the Gardens in July 2017 The numbers refer to the gardens as shown on your map. There is a great deal of interest in the Garden this month. In particular, as you wander around the Garden, look out for the tall, spiky flower of the Puyas. This is the flower that is depicted on the Garden logo (which appears at the bottom of this article) and we hold the national collection of this strange group of plants. These very spiky leaved plants are relatives of Bromeliads and they are native to the Andes mountains in South America. At this time of year, a number of them are coming into flower. The fierce, spiky rosette of leaves deters browsing animals. Indeed, sheep have been found dead having become caught up in the barbs ands unable to escape. It may be that, as they rot down, they add nutrients to the soil that benefit the Puya plants. The flower spikes have many branches ending in pointed spikes. These spikes are used as perching posts for native birds which collect nectar from the flowers, thereby pollinating them. Look for Puyas particularly in the American Garden (Near the Totem Pole) and the Arid Garden (16). Puya chilensis has yellow flowers (Left below); Puya berteroniana (Right below) has jade green flowers. However, many of the plants in the Garden are hybrids. Left: Puya chilensis with chartreuse flowers Right: Puya berteroniana with jade flowers Soon after entering the Garden, the South African Terrace (3) is now a riot of colour. There are Pelargoniums, Osteospermums, yellow daisy bushes (Euryops), pink African mallows (Anisodontea), blue African Corn Lilies (Agapanthus) and many more, each represented by different species and cultivars. -

The Vulnerable and Endangered Plants of Xishuangbanna
The Vulnerable and Endangered Plants of Xishuang- banna Prefecture, Yunnan Province, China Zou Shou-qing Efforts are now being taken to preserve endangered species in the rich tropical flora of China’s "Kingdom of Plants and Animals" Xishuangbanna Prefecture is a tropical area of broadleaf forest-occurs in Xishuangbanna. China situated in southernmost Yunnan Coniferous forest develops above 1,200 me- Province, on the border with Laos and Burma. ters. In addition, Xishuangbanna lies at the Lying between 21°00’ and 21°30’ North Lati- transitional zone between the floras of Ma- tude and 99°55’ and 101°15’ East Longitude, laya, Indo-Himalaya, and South China and the prefecture occupies 19,220 square kilo- therefore boasts a great number of plant spe- meters of territory. It attracts Chinese and cies. So far, about 4,000 species of vascular non-Chinese botanists alike and is known plants have been identified. This means that popularly as the "Kingdom of Plants and Xishuangbanna, an area occupying only 0.22 Animals." The Langchan River passes percent of China, supports about 12 percent through its middle. of the species in China’s flora. The species be- Xishuangbanna is very hilly, about 95 per- long to 1,471 genera in 264 families and in- cent of its terrain being hills and low, undu- clude 262 species of ferns in 94 genera and 47 lating mountains that reach 500 to 1,500 families, 25 species of gymnosperms in 12 meters in elevation. The highest peak is 2,400 genera and 9 families, and 3,700 species of meters in elevation. -
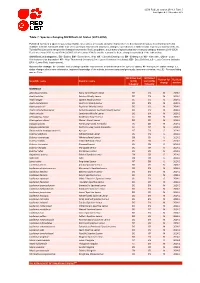
Table 7: Species Changing IUCN Red List Status (2013-2014)
IUCN Red List version 2014.3: Table 7 Last Updated: 13 November 2014 Table 7: Species changing IUCN Red List Status (2013-2014) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2013 (IUCN Red List version 2013.2) and 2014 (IUCN Red List version 2014.3) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2013) List (2014) change version Category Category MAMMALS Allocebus trichotis Hairy-eared Dwarf Lemur DD VU N 2014.1 Avahi betsileo Betsileo Woolly Lemur -
T H E P L a N T H U N T E R S Plantejægerne JAGTEN PÅ DET
T h e P l a n t H u n t e r s Plantejægerne JAGTEN PÅ DET GRØNNE GULD Preben Escherich Holkjær Den allerførste gang, planteindsamling blev nævnt, er for hele 3.000 år siden og hænger sammen med krig og erobring. Et eksempel er den ægyptiske dronning Hatshepsut (1490-1469 f.kr.), der erobrede Nubien og som bl.a. fik sine soldater til at bringe myrra med tilbage til Ægypten. Der findes også et brev fra den assyriske konge, Tiglat Pilesar I (ca. 1100 f.kr.), hvori han stolt berettede, at han havde hjemført såvel ceder som buksbom fra de lande, han havde erobret. En anden vidt berømt feltherre var Alexander den Store. Alexander havde som ung været elev hos den navnkundige Aristoteles. Da han senere begav sig ud på erobrings- togter, skal han efter sigende have sendt bl.a. planter og dyr til sin tidligere læreme- ster. Nogle af de planter, som Alexander opdagede, var citron og banan. Romerne, som var meget havebevidste, tog også planter med hjem fra deres kolonier, ligesom de bragte hjemlige planter med sig til de lande, de erobrede og besatte. I Kina har man allerede nævnt rhododendron i det 5. århundrede. Pæonen var en vigtig plante i mytologien. Den var opkaldt efter Pæeon, der var læge for guderne på Olympen. Pæonen er blevet brugt som medicinsk plante i mindst 2000 år. Den er også blevet brugt i parfumefremstillingen. Desuden har man anvendt den under fremstilling af farvestof. De første, der fik øjnene op for pæoner, var kineserne. Man har dyrket pæoner i Kina i de sidste 1500 år.