Midtermexam1fall 2012
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Interview with John Gurdon Aidan Maartens*,‡
© 2017. Published by The Company of Biologists Ltd | Development (2017) 144, 1581-1583 doi:10.1242/dev.152058 SPOTLIGHT An interview with John Gurdon Aidan Maartens*,‡ John Gurdon is a Distinguished Group Leader in the Wellcome Trust/ Cancer Research UK Gurdon Institute and Professor Emeritus in the Department of Zoology at the University of Cambridge. In 2012, he was awarded the Nobel Prize in Physiology or Medicine jointly with Shinya Yamanaka for work on the reprogramming of mature cells to pluripotency, and his lab continues to investigate the molecular mechanisms of nuclear reprogramming by oocytes and eggs. We met John in his Cambridge office to discuss his career and hear his thoughts on the past, present and future of reprogramming. Your first paper was published in 1954 and concerned not embryology but entomology. How did that come about? Well, that early paper was published in the Entomologist’s Monthly Magazine. Throughout my early life, I really was interested in insects, and used to collect butterflies and moths. When I was an undergraduate I liked to take time off and go out to Wytham Woods near Oxford to see what I could find. So I went out one cold spring day and there were no butterflies about, nor any moths, but, out of nowhere, there was a fly – I caught it, put it in my bottle, and had a look at it. The first thing that was obvious was that it wasn’t a fly, it was a hymenopteran, but when I tried to identify it I simply could cells in the body have the same genes. -

2004 Albert Lasker Nomination Form
albert and mary lasker foundation 110 East 42nd Street Suite 1300 New York, ny 10017 November 3, 2003 tel 212 286-0222 fax 212 286-0924 Greetings: www.laskerfoundation.org james w. fordyce On behalf of the Albert and Mary Lasker Foundation, I invite you to submit a nomination Chairman neen hunt, ed.d. for the 2004 Albert Lasker Medical Research Awards. President mrs. anne b. fordyce The Awards will be offered in three categories: Basic Medical Research, Clinical Medical Vice President Research, and Special Achievement in Medical Science. This is the 59th year of these christopher w. brody Treasurer awards. Since the program was first established in 1944, 68 Lasker Laureates have later w. michael brown Secretary won Nobel Prizes. Additional information on previous Lasker Laureates can be found jordan u. gutterman, m.d. online at our web site http://www.laskerfoundation.org. Representative Albert Lasker Medical Research Awards Program Nominations that have been made in previous years may be updated and resubmitted in purnell w. choppin, m.d. accordance with the instructions on page 2 of this nomination booklet. daniel e. koshland, jr., ph.d. mrs. william mccormick blair, jr. the honorable mark o. hatfied Nominations should be received by the Foundation no later than February 2, 2004. Directors Emeritus A distinguished panel of jurors will select the scientists to be honored. The 2004 Albert Lasker Medical Research Awards will be presented at a luncheon ceremony given by the Foundation in New York City on Friday, October 1, 2004. Sincerely, Joseph L. Goldstein, M.D. Chairman, Awards Jury Albert Lasker Medical Research Awards ALBERT LASKER MEDICAL2004 RESEARCH AWARDS PURPOSE AND DESCRIPTION OF THE AWARDS The major purpose of these Awards is to recognize and honor individuals who have made signifi- cant contributions in basic or clinical research in diseases that are the main cause of death and disability. -

2019 Annual Report
BECKMAN CENTER 279 Campus Drive West Stanford, CA 94305 650.723.8423 Stanford University | Beckman Center 2019 Annual Report Annual 2019 | Beckman Center University Stanford beckman.stanford.edu 2019 ANNUAL REPORT ARNOLD AND MABEL BECKMAN CENTER FOR MOLECULAR AND GENETIC MEDICINE 30 Years of Innovation, Discovery, and Leadership in the Life Sciences CREDITS: Cover Design: Neil Murphy, Ghostdog Design Graphic Design: Jack Lem, AlphaGraphics Mountain View Photography: Justin Lewis Beckman Center Director Photo: Christine Baker, Lotus Pod Designs MESSAGE FROM THE DIRECTOR Dear Friends and Trustees, It has been 30 years since the Beckman Center for Molecular and Genetic Medicine at Stanford University School of Medicine opened its doors in 1989. The number of translational scientific discoveries and technological innovations derived from the center’s research labs over the course of the past three decades has been remarkable. Equally remarkable have been the number of scientific awards and honors, including Nobel prizes, received by Beckman faculty and the number of young scientists mentored by Beckman faculty who have gone on to prominent positions in academia, bio-technology and related fields. This year we include several featured articles on these accomplishments. In the field of translational medicine, these discoveries range from the causes of skin, bladder and other cancers, to the identification of human stem cells, from the design of new antifungals and antibiotics to the molecular underpinnings of autism, and from opioids for pain -

Profile of John Gurdon and Shinya Yamanaka, 2012 Nobel Laureates in Medicine Or Physiology
PROFILE PROFILE Profile of John Gurdon and Shinya Yamanaka, 2012 Nobel Laureates in Medicine or Physiology Alan Colman1 Executive Director, Singapore Stem Cell Consortium, A*STAR Institute of Medical Biology We celebrate the 2012 Nobel Prize for genes and it was selective gene expression Medicine awarded to Sir John Gurdon and that accounted for cell fate choices. Al- Shinya Yamanaka for their groundbreaking though the initial “cloning” experiments contributions to the field of cell reprogram- generated swimming tadpoles, it wasn’t un- ming. In 1962, in a series of experiments til Gurdon showed that these tadpoles could inspired by Briggs and King (1), Gurdon mature into fertile adults (4) that it became demonstrated that the nucleus of a frog clear that the frog somatic cell nucleus con- somatic cell could be reprogrammed to be- tained all of the genes needed for full de- Sir John B. Gurdon (Left) and Shinya Yamanaka have like the nucleus of a fertilized frog egg velopment. The technical inefficiencies of (Right) during an interview with Nobelprize.org (2). By inserting the nuclei of intestinal ep- his experiments begged the question of ithelial cells into enucleated eggs, Gurdon whether the frogs created by Gurdon using on December 6, 2012. Image Copyright Nobel was able to create healthy swimming tad- SCNT in fact arose from unspecialized cell Media AB 2012/Niklas Elmehed. poles. These experiments were the first suc- donors present in the epithelial population. cessful instances of somatic cell nuclear Such doubts were dispelled when skin nu- The series of reprogramming successes transfer (SCNT) using genetically normal clei that were demonstrably specialized were that Gurdon’s work inspired involved radical cells. -

ILAE Historical Wall02.Indd 10 6/12/09 12:04:44 PM
2000–2009 2001 2002 2003 2005 2006 2007 2008 Tim Hunt Robert Horvitz Sir Peter Mansfi eld Barry Marshall Craig Mello Oliver Smithies Luc Montagnier 2000 2000 2001 2002 2004 2005 2007 2008 Arvid Carlsson Eric Kandel Sir Paul Nurse John Sulston Richard Axel Robin Warren Mario Capecchi Harald zur Hauser Nobel Prizes 2000000 2001001 2002002 2003003 200404 2006006 2007007 2008008 Paul Greengard Leland Hartwell Sydney Brenner Paul Lauterbur Linda Buck Andrew Fire Sir Martin Evans Françoise Barré-Sinoussi in Medicine and Physiology 2000 1st Congress of the Latin American Region – in Santiago 2005 ILAE archives moved to Zurich to become publicly available 2000 Zonismide licensed for epilepsy in the US and indexed 2001 Epilepsia changes publishers – to Blackwell 2005 26th International Epilepsy Congress – 2001 Epilepsia introduces on–line submission and reviewing in Paris with 5060 delegates 2001 24th International Epilepsy Congress – in Buenos Aires 2005 Bangladesh, China, Costa Rica, Cyprus, Kazakhstan, Nicaragua, Pakistan, 2001 Launch of phase 2 of the Global Campaign Against Epilepsy Singapore and the United Arab Emirates join the ILAE in Geneva 2005 Epilepsy Atlas published under the auspices of the Global 2001 Albania, Armenia, Arzerbaijan, Estonia, Honduras, Jamaica, Campaign Against Epilepsy Kyrgyzstan, Iraq, Lebanon, Malta, Malaysia, Nepal , Paraguay, Philippines, Qatar, Senegal, Syria, South Korea and Zimbabwe 2006 1st regional vice–president is elected – from the Asian and join the ILAE, making a total of 81 chapters Oceanian Region -

Lasker Interactive Research Nom'18.Indd
THE 2018 LASKER MEDICAL RESEARCH AWARDS Nomination Packet albert and mary lasker foundation November 1, 2017 Greetings: On behalf of the Albert and Mary Lasker Foundation, I invite you to submit a nomination for the 2018 Lasker Medical Research Awards. Since 1945, the Lasker Awards have recognized the contributions of scientists, physicians, and public citizens who have made major advances in the understanding, diagnosis, treatment, cure, and prevention of disease. The Medical Research Awards will be offered in three categories in 2018: Basic Research, Clinical Research, and Special Achievement. The Lasker Foundation seeks nominations of outstanding scientists; nominations of women and minorities are encouraged. Nominations that have been made in previous years are not automatically reconsidered. Please see the Nomination Requirements section of this booklet for instructions on updating and resubmitting a nomination. The Foundation accepts electronic submissions. For information on submitting an electronic nomination, please visit www.laskerfoundation.org. Lasker Awards often presage future recognition of the Nobel committee, and they have become known popularly as “America’s Nobels.” Eighty-seven Lasker laureates have received the Nobel Prize, including 40 in the last three decades. Additional information on the Awards Program and on Lasker laureates can be found on our website, www.laskerfoundation.org. A distinguished panel of jurors will select the scientists to be honored with Lasker Medical Research Awards. The 2018 Awards will -
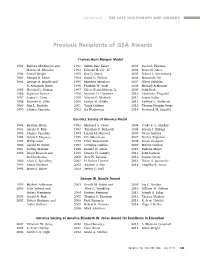
Previous Recipients of GSA Awards
GENETICS | THE 2015 GSA HONORS AND AWARDS Previous Recipients of GSA Awards Thomas Hunt Morgan Medal 1981 Barbara McClintock and 1991 Armin Dale Kaiser 2003 David S. Hogness Marcus M. Rhoades 1992 Edward H. Coe, Jr. 2004 Bruce N. Ames 1982 Sewall Wright 1993 Ray D. Owen 2005 Robert L. Metzenberg 1983 Edward B. Lewis 1994 David D. Perkins 2006 Masatoshi Nei 1984 George W. Beadle and 1995 Matthew Meselson 2007 Oliver Smithies R. Alexander Brink 1996 Franklin W. Stahl 2008 Michael Ashburner 1985 Herschel L. Roman 1997 Oliver Evans Nelson, Jr. 2009 John Roth 1986 Seymour Benzer 1998 Norman H. Horowitz 2010 Alexander Tzagoloff 1987 James F. Crow 1999 Salome G. Waelsch 2011 James Haber 1988 Norman H. Giles 2000 Evelyn M. Witkin 2012 Kathryn V. Anderson 1989 Dan L. Lindsley 2001 Yasuji Oshima 2013 Thomas Douglas Petes 1990 Charles Yanofsky 2002 Ira Herskowitz 2014 Frederick M. Ausubel Genetics Society of America Medal 1981 Beatrice Mintz 1992 Maynard V. Olson 2004 Trudy F. C. Mackay 1982 Gerald R. Fink 1993 Jonathan R. Beckwith 2005 Steven J. Elledge 1983 Charles Yanofsky 1994 Leland H. Hartwell 2006 Victor Ambros 1984 David S. Hogness 1995 Eric Wieschaus 2007 Shirley Tilghman 1985 Philip Leder 1996 Elliot Meyerowitz 2008 Susan Lindquist 1986 Gerald M. Rubin 1997 Christine Guthrie 2009 Marian Carlson 1987 Sydney Brenner 1998 Ronald W. Davis 2010 Barbara Meyer 1988 David Botstein and 1999 Charles H. Langley 2011 John Carlson Ira Herskowitz 2000 Jack W. Szostak 2012 Joanne Chory 1989 Allan C. Spradling 2001 H. Robert Horvitz 2013 Elaine A. Ostrander 1990 Nancy Kleckner 2002 Andrew Z. -

Loss of Krüppel-Like Factor 6 Cripples Podocyte Mitochondrial Function
Loss of Krüppel-like factor 6 cripples podocyte mitochondrial function Jeffrey B. Kopp J Clin Invest. 2015;125(3):968-971. https://doi.org/10.1172/JCI80280. Commentary Krüppel-like factors (KLFs) are zinc finger transcription factors that share homology in three C-terminal zinc finger domains. KLF family members are expressed in most if not all tissues and have diverse roles in organismal development and cell differentiation, function, and death. The glomerular podocyte is particularly sensitive to mitochondrial dysfunction, as seen in various genetic disorders manifesting as progressive glomerulosclerosis. In this issue of the JCI, Mallipattu and coworkers show that KLF6 expression is reduced in mouse and human glomerular disease. Podocyte-specific deletion of Klf6 expression in mice leads to mitochondrial dysfunction and apoptosis, followed by glomerulosclerosis. This is the first demonstration that defective transcriptional regulation of nuclear-encoded mitochondrial genes can result in experimental glomerular disease. Find the latest version: https://jci.me/80280/pdf COMMENTARY The Journal of Clinical Investigation Loss of Krüppel-like factor 6 cripples podocyte mitochondrial function Jeffrey B. Kopp Kidney Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), NIH, Bethesda, Maryland, USA. activators, and group 3 KLFs bind and Krüppel-like factors (KLFs) are zinc finger transcription factors that share potentiate the activity of the co-repres- sor SIN3A; KLF16 and -17 do not cluster homology in three C-terminal zinc finger domains. KLF family members with other group members (ref. 6 and Fig- are expressed in most if not all tissues and have diverse roles in organismal ure 2). -

Embryo Tanulmányozási Módszerek
Methods in developmental biology Dr. Nandor Nagy Developmental model organisms Often used model organisms in developmental biology include the following: Vertebrates Zebrafish Danio rario Medakafish Oryzias latipes Fugu (pufferfish) Takifugu rubripes Frog Xenopus laevis, Xenopus tropicalis Chicken Gallus gallus Mouse Mus musculus (Mammalian embryogenesis) Invertebrates Lancelet Branchiostoma lanceolatum Ascidian Ciona intestinalis Sea urchin Strongylocentrotus purpuratus Roundworm Caenorhabditis elegans Fruit fly Drosophila melanogaster (Drosophila embryogenesis) Plants (Plant embryogenesis) Arabidopsis thaliana Maize Snapdragon Antirrhinum majus Other Slime mold Dictyostelium discoideum Induction The Nobel Prize in Physiology or Medicine 1935 was awarded to Hans Spemann "for his discovery of the organizer effect in embryonic development". 2002 Nobel prize Psysiology and Medicine: Sydney Brenner, John E. Sulston, H. Robert Horovitz By establishing and using the nematode Caenorhabditis elegans as an experimental model system, possibilities were opened to follow cell division and differentiation from the fertilized egg to the adult. The Laureates have identified key genes regulating organ development and programmed cell death and have shown that corresponding genes exist in higher species, including man. The discoveries are important for medical research and have shed new light on the pathogenesis of many diseases. 2019 Lasker Awards highlight the invaluable role of animal research The Lasker Awards are among the most prestigious prizes -

Storia Di Una Rana Francesca Buoninconti
micron / storia della scienza Storia di una rana Francesca Buoninconti La scienza è continuamente e dovessimo immaginare una vita Ma cominciamo dall’inizio. La rana S esaltante e piena di avventure, pro- artigliata africana è una specie origi- contraddistinta dalla ricerca babilmente ci immagineremmo quella naria dell’Africa meridionale, diffusa degli strumenti più adatti per di un astronauta. O di uno scienziato dall’Angola al Sudafrica. Ed è sempre che ha ricevuto il premio Nobel per rimasta lì, praticamente indisturbata, affrontare l’indagine scienti- le sue scoperte. O ancora di un im- fino agli inizi degli anni Trenta del fica: dai piselli di Mendel, al portante diplomatico. E perché no, Novecento, fino a quando, cioè, ha potremmo immedesimarci in qualche risvegliato l’interesse di un gruppo di riccio di mare passando per personaggio del passato, che ha lascia- scienziati che lavorava in Sudafrica: l’assone gigante del calamaro. to un segno nella storia. Di sicuro a l’inglese Lancillotto Thomas Hogben nessuno verrebbe in mente che tutto e i sudafricani Hillel Abbe Shapiro e Tra questi la rana artigliata questo potrebbe essere racchiuso nella Herry Zwarenstein. Saranno loro a africana si è ritagliata pagine storia di un animale, o meglio di una rendere lo xenopo liscio famoso in tut- specie che è diventa protagonista della to il mondo nel giro di qualche anno. importanti nei libri di medici- ricerca medica, ha viaggiato in lungo È infatti l’ottobre del 1933 quando, na e biologia e in largo per i continenti, è stata tra i in un rapporto alla Royal Society precursori degli esperimenti di clona- del Sudafrica, Shapiro e Zwarenstein zione e sulle cellule staminali e addi- annunciano che il mese precedente rittura è riuscita a imbarcarsi a bordo hanno condotto con successo ben 35 di uno Space Shuttle. -

Professor Oliver Smithies Was Born on June 23, 1925, in Halifax, England, and As a Child Went to a Small School in Copley Village Near to Halifax
Professor Oliver Smithies was born on June 23, 1925, in Halifax, England, and as a child went to a small school in Copley village near to Halifax. Subsequently he was a student at Heath Grammar School in Halifax until he was awarded a scholarship from Balliol College, Oxford University. At Oxford he received a Bachelor of Arts Degree in Physiology with First Class Honors in 1946. In 1951, he obtained his M.A. and D.Phil. degrees in Biochemistry from Oxford. Professor Smithies then moved to the United States as a Postdoctoral Fellow in Physical Chemistry at the University of Wisconsin. After two years at Wisconsin, Professor Smithies accepted a position at the Connaught Medical Research Laboratories in Toronto and stayed there from 1953 to 1960, first as a Research Assistant and then as a Research Associate. In 1960, Professor Smithies returned to Wisconsin as Assistant Professor of Medical Genetics and Genetics, advancing to Full Professor by 1963. He was named the Leon J. Cole Professor in 1971. In 1988, he joined the Department of Pathology at the University of North Carolina, as the Excellence Professor of Pathology. He is now the Weatherspoon Eminent Distinguished Professor of Pathology and Laboratory Medicine at UNC, and remains an actively engaged scientist working at the bench in the laboratory he shares with his wife, Nobuyo Maeda who is the Robert H. Wagner Distinguished Professor of Pathology and Laboratory Medicine. In the mid-1950s, Professor Smithies described the first high resolution electrophoresis system (starch gel), and with it he discovered that normal humans have unsuspected inherited differences in their proteins. -

Close to the Edge: Co-Authorship Proximity of Nobel Laureates in Physiology Or Medicine, 1991 - 2010, to Cross-Disciplinary Brokers
Close to the edge: Co-authorship proximity of Nobel laureates in Physiology or Medicine, 1991 - 2010, to cross-disciplinary brokers Chris Fields 528 Zinnia Court Sonoma, CA 95476 USA fi[email protected] January 2, 2015 Abstract Between 1991 and 2010, 45 scientists were honored with Nobel prizes in Physiology or Medicine. It is shown that these 45 Nobel laureates are separated, on average, by at most 2.8 co-authorship steps from at least one cross-disciplinary broker, defined as a researcher who has published co-authored papers both in some biomedical discipline and in some non-biomedical discipline. If Nobel laureates in Physiology or Medicine and their immediate collaborators can be regarded as forming the intuitive “center” of the biomedical sciences, then at least for this 20-year sample of Nobel laureates, the center of the biomedical sciences within the co-authorship graph of all of the sciences is closer to the edges of multiple non-biomedical disciplines than typical biomedical researchers are to each other. Keywords: Biomedicine; Co-authorship graphs; Cross-disciplinary brokerage; Graph cen- trality; Preferential attachment Running head: Proximity of Nobel laureates to cross-disciplinary brokers 1 1 Introduction It is intuitively tempting to visualize scientific disciplines as spheres, with highly produc- tive, well-funded intellectual and political leaders such as Nobel laureates occupying their centers and less productive, less well-funded researchers being increasingly peripheral. As preferential attachment mechanisms as well as the economics of employment tend to give the well-known and well-funded more collaborators than the less well-known and less well- funded (e.g.