Nanoremediation Technologies for Sustainable Remediation of Contaminated
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Projected Roles for Disruptive and Emergent Technologies
50 Technology as a Driver of Future Change in the Forest Sector Technology as a Driver of Future Change in the Forest Sector: Projected Roles for Disruptive and Emergent Technologies George H. Kubik Abstract: This paper examines emergent and disruptive technologies as potential drivers of change in forest sector futures. Two questions are addressed: (1) Which emergent and disruptive technologies can be projected to substantively impact forestry futures? (2) What are the possible implications of emergent and disruptive technologies for decision makers, policymakers, and other stakeholders involved in forest sector futures? A 20-year timeframe is used for this explorative paper. A cross-disciplinary review of futures literature was implemented to identify and investigate leading emergent and disruptive technologies. A list of candidate technologies was developed from the literature review and eight technologies were selected: artificial intelligence, autonomous vehicles, electronic performance enhancement systems, genomics and synthetic biology, the Internet of Things, materials science, nanotechnology, and robotics. Each of the eight technologies was then defined and three representative forecasts were projected for each technology. The goal is to provide decision makers, policymakers, and other stakeholders in the forest sector with an awareness of emergent and potentially disruptive technologies and how they might disrupt forest sector futures. The purpose of this paper is not to predict the future in detail, but to (1) promote awareness and informed thinking about the relationship between potentially disruptive technologies and forest sector futures and (2) stimulate a research agenda based on the study of these projected futures. KEY WORDS: emergent technology, disruptive technology, artificial intelligence, autonomous vehicles, electronic performance enhancement systems, genomics and synthetic biology, Internet of Things, materials science, nanotechnology and robotics Citation: Kubik, George H. -

The Nanotoxicology of a Newly Developed Zero-Valent Iron
The nanotoxicology of a newly developed zero-valent iron nanomaterial for groundwater remediation and its remediation efficiency assessment combined with in vitro bioassays for detection of dioxin-like environmental pollutants Von der Fakultät für Mathematik, Informatik und Naturwissenschaften der RWTH Aachen University zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigte Dissertation vorgelegt von Diplom-Biologe Andreas Herbert Schiwy aus Tarnowitz (Polen) Berichter: Universitätsprofessor Dr. rer. nat. Henner Hollert Universitätsprofessor Dr. rer. nat. Andreas Schäffer Tag der mündlichen Prüfung 28. Juli 2016 Diese Dissertation ist auf den Internetseiten der Universitätsbibliothek online verfügbar. To my wife and my children Summary Summary The assessment of chemicals and new compounds is an important task of ecotoxicology. In this thesis a newly developed zero-valent iron material for nanoremediation of groundwater contaminations was investigated and in vitro bioassays for high throughput screening were developed. These two elements of the thesis were combined to assess the remediation efficiency of the nanomaterial on the groundwater contaminant acridine. The developed in vitro bioassays were evaluated for quantification of the remediation efficiency. Within the NAPASAN project developed iron based nanomaterial showed in a model field application its nanoremediation capabilities to reduce organic contaminants in a cost effective way. The ecotoxicological evaluation of the nanomaterial in its reduced and oxidized form was conducted with various ecotoxicological test systems. The effects of the reduced nanomaterial with field site resident dechlorinating microorganisms like Dehalococcoides sp., Desulfitobacterium sp., Desulfomonile tiedjei, Dehalobacter sp., Desulfuromonas sp. have been investigated in batch und column experiments. A short-term toxicity of the reduced nanomaterial was shown. -

Nanodata Landscape Compilation
NanoData Landscape Compilation Environment Written by the Joint Institute for Innovation Policy, Brussels, Belgium, in co-operation with CWTS, University of Leiden, Leiden, Netherlands; Frost & Sullivan Limited, London, United Kingdom; Joanneum Research Forschungsgesellschaft mbH, Graz, Austria; the Nanotechnology Industries Association, Brussels, Belgium; Tecnalia Research and Innovation, Bilbao, Spain; and TNO, The Hague, Netherlands. August 2016 EUROPEAN COMMISSION Directorate-General for Research and Innovation Directorate Industrial Technologies Unit D.3 - Advanced Materials and Nanotechnologies E-mail: [email protected] European Commission B-1049 Brussels EUROPEAN COMMISSION NanoData Landscape Compilation Environment Written by: Jacqueline E M Allan Babette Bakker Harrie Buist Guillaume Flament Christian Hartmann Iain Jawad Eelco Kuijpers Hanna Kuittinen Ed Noyons Claire Stolwijk Xabier Uriarte Olaeta and Alfredo Yegros Additional contributions: Ashfeen Aribea Iker Barrondo Saez Robbert Fisher Milica Misojcic Luca Remotti Directorate-General for Research and Innovation 2017 Key Enabling Technologies EN EUROPE DIRECT is a service to help you find answers to your questions about the European Union Freephone number (*): 00 800 6 7 8 9 10 11 (*) The information given is free, as are most calls (though some operators, phone boxes or hotels may charge you) LEGAL NOTICE This document has been prepared for the European Commission however it reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein. More information on the European Union is available on the internet (http://europa.eu). Luxembourg: Publications Office of the European Union, 2017. PDF ISBN 978-92-79-68388-6 doi: 10.2777/017097 KI-02-17-427-EN-N © European Union, 2017. -

Smart Materials and Nanotechnology March 16-17, 2020 | Frankfurt, Germany
7th International Conference on Smart Materials and Nanotechnology March 16-17, 2020 | Frankfurt, Germany Hosting Organization: Pulsus Group 40 Bloomsbury Way | Lower Ground Floor London, United Kingdom | WC1A 2SE | Tel: +1-408-429-2646 [email protected] https://smart-advanced-materials.pulsusconference.com/ Invitation PULSUS brings in a new spin on conferences by presenting the latest scientific improvements in your field. Listen to motivating keynotes from thought leaders, or rub elbows with pioneers across the globe. Frankfurt is all set for an amazing event as PULSUS proudly presents the “7th International Conference on Smart Materials and Nanotechnology” slated on March 16-17, 2020, Frankfurt, Germany. PULSUS cordially welcome researchers, academicians, students and business professionals in the field of Nanotechnology professionals from around the world to participate in the upcoming Smart Materials 2020. The meeting for this year will revolve around the theme “Emerging trends in the fields of Smart Materials and Nanotechnology” thus relaying the most cutting edge findings in the field of Smart Materials and Research. The two day meeting is going to be an event to look forward to for its enlightening symposiums & workshops from established experts of the field, exceptional keynote sessions directed by the best in the business. It will also prove to be a brilliant open door for the representatives from Universities and Institutes to cooperate with the world class researchers and an outstanding opportunity for businesses keen at expanding their global market reach. Interested individuals can confirm their participation by registering for the conference along with their colleagues. Register soon and avail exciting early bird discounts. -

Ecotoxicology and Environmental Safety 154 (2018) 237–244
Ecotoxicology and Environmental Safety 154 (2018) 237–244 Contents lists available at ScienceDirect Ecotoxicology and Environmental Safety journal homepage: www.elsevier.com/locate/ecoenv Ecofriendly nanotechnologies and nanomaterials for environmental T applications: Key issue and consensus recommendations for sustainable and ecosafe nanoremediation ⁎ I. Corsia, ,1, M. Winther-Nielsenb, R. Sethic, C. Puntad, C. Della Torree, G. Libralatof, G. Lofranog, ⁎ L. Sabatinih, M. Aielloi, L. Fiordii, F. Cinuzzij, A. Caneschik, D. Pellegrinil, I. Buttinol, ,1 a Department of Physical, Earth and Environmental Sciences, University of Siena, via Mattioli, 4-53100 Siena, Italy b Department of Environment and Toxicology, DHI, Agern Allé 5, 2970 Hoersholm, Denmark c Department of Environment, Land and Infrastructure Engineering (DIATI), Politecnico di Torino, Italy d Department of Chemistry, Materials, and Chemical Engineering “G. Natta”, Politecnico di Milano and RU INSTM, Via Mancinelli 7, 20131 Milano, Italy e Department of Bioscience, University of Milano, via Celoria 26, 20133 Milano, Italy f Department of Biology, University of Naples Federico II, via Cinthia ed. 7, 80126 Naples, Italy g Department of Chemical and Biology “A. Zambelli”, University of Salerno, via Giovanni Paolo II 132, 84084 Fisciano, SA, Italy h Regional Technological District for Advanced Materials, c/o ASEV SpA (management entity), via delle Fiascaie 12, 50053 Empoli, FI, Italy i Acque Industriali SRL, Via Molise, 1, 56025 Pontedera, PI, Italy j LABROMARE SRL, Via dell'Artigianato -

Use of Nanotechnology for the Bioremediation of Contaminants: a Review
processes Review Use of Nanotechnology for the Bioremediation of Contaminants: A Review Edgar Vázquez-Núñez 1,* , Carlos Eduardo Molina-Guerrero 1, Julián Mario Peña-Castro 2, Fabián Fernández-Luqueño 3 and Ma. Guadalupe de la Rosa-Álvarez 1 1 Departamento de Ingenierías Química, Electrónica y Biomédica, División de Ciencias e Ingenierías, Campus León, Universidad de Guanajuato, Lomas del Bosque 103, Lomas del Campestre, León, Guanajuato C.P. 37150, Mexico; cmolina@fisica.ugto.mx (C.E.M.-G.); [email protected] (M.G.d.l.R.-Á.) 2 Laboratorio de Biotecnología Vegetal, Instituto de Biotecnología, Universidad del Papaloapan, Tuxtepec, Oaxaca C.P. 68333, Mexico; [email protected] 3 Programas en Sustentabilidad de los Recursos Naturales y Energía, Cinvestav Saltillo Industrial, Parque Industrial, Ramos Arizpe, Coahuila C.P. 25900, Mexico; [email protected] * Correspondence: edgarvqznz@fisica.ugto.mx Received: 22 May 2020; Accepted: 8 July 2020; Published: 13 July 2020 Abstract: Contaminants, organic or inorganic, represent a threat for the environment and human health and in recent years their presence and persistence has increased rapidly. For this reason, several technologies including bioremediation in combination with nanotechnology have been explored to identify more systemic approaches for their removal from environmental matrices. Understanding the interaction between the contaminant, the microorganism, and the nanomaterials (NMs) is of crucial importance since positive and negative effects may be produced. For example, some nanomaterials are stimulants for microorganisms, while others are toxic. Thus, proper selection is of paramount importance. The main objective of this review was to analyze the principles of bioremediation assisted by nanomaterials, nanoparticles (NPs) included, and their interaction with environmental matrices. -

EPA Nanotechnology White Paper EXTERNAL REVIEW DRAFT 12-2-…
December 2, 2005 External Review Draft U.S. Environmental Protection Agency EXTERNAL REVIEW DRAFT Nanotechnology White Paper Prepared for the U.S. Environmental Protection Agency by members of the Nanotechnology Workgroup, a group of EPA’s Science Policy Council Science Policy Council U.S. Environmental Protection Agency Washington, DC 20460 NOTICE This document is an external review draft. It has not been formally released by the U.S. Environmental Protection Agency and should not at this stage be construed to represent Agency position. Draft Nanotechnology White Paper – External Review Draft DISCLAIMER Mention of trade names or commercial products does not constitute endorsement of recommendation for use. Note: This is an external review draft, and is not approved for final publication. ii Draft Nanotechnology White Paper – External Review Draft Nanotechnology White Paper Workgroup Co-Chairs Jeff Morris Jim Willis Office of Research and Development Office of Prevention, Pesticides and Toxic Substances Science Policy Council Staff Kathryn Gallagher Office of the Science Advisor Subgroup Co-Chairs External Coordination Ecological Effects Risk Management Steve Lingle, ORD Anne Fairbrother, ORD Flora Chow, OPPT Dennis Utterback, ORD Vince Nabholz, OPPTS EPA Research Strategy Human Exposures Converging Technologies Barbara Karn, ORD Scott Prothero, OPPT Nora Savage, ORD Risk Assessment Environmental Fate Pollution Prevention Phil Sayre, OPPTS John Scalera, OEI Walter Schoepf, Region 2 Bob Boethling, OPPTS Physical-Chemical Environmental -

Recent Advances of Nanoremediation Technologies for Soil and Groundwater Remediation: a Review
water Review Recent Advances of Nanoremediation Technologies for Soil and Groundwater Remediation: A Review Motasem Y. D. Alazaiza 1,* , Ahmed Albahnasawi 2 , Gomaa A. M. Ali 3 , Mohammed J. K. Bashir 4 , Nadim K. Copty 5 , Salem S. Abu Amr 6 , Mohammed F. M. Abushammala 7 and Tahra Al Maskari 1 1 Department of Civil and Environmental Engineering, College of Engineering, A’Sharqiyah University, Ibra 400, Oman; [email protected] 2 Department of Environmental Engineering-Water Center (SUMER), Gebze Technical University, Kocaeli 41400, Turkey; [email protected] 3 Chemistry Department, Faculty of Science, Al-Azhar University, Assiut 71524, Egypt; [email protected] 4 Department of Environmental Engineering, Faculty of Engineering and Green Technology (FEGT), Universiti Tunku Abdul Rahman, Kampar 31900, Malaysia; [email protected] 5 Institute of Environmental Sciences, Bogazici University, Istanbul 34342, Turkey; [email protected] 6 Faculty of Engineering, Demir Campus, Karabuk University, Karabuk 78050, Turkey; [email protected] 7 Department of Civil Engineering, Middle East College, Knowledge Oasis Muscat, Muscat 135, Oman; [email protected] * Correspondence: [email protected] Abstract: Nanotechnology has been widely used in many fields including in soil and groundwater remediation. Nanoremediation has emerged as an effective, rapid, and efficient technology for Citation: Alazaiza, M.Y.D.; soil and groundwater contaminated with petroleum pollutants and heavy metals. This review Albahnasawi, A.; Ali, G.A.M.; Bashir, provides an overview of the application of nanomaterials for environmental cleanup, such as soil M.J.K.; Copty, N.K.; Amr, S.S.A.; and groundwater remediation. Four types of nanomaterials, namely nanoscale zero-valent iron Abushammala, M.F.M.; Al Maskari, T. -

Importance of Nanotechnology for Environmental Related Issues
International Journal of Science and Research (IJSR) ISSN: 2319-7064 SJIF (2019): 7.583 Importance of Nanotechnology for Environmental Related Issues Vinod Kumar Singh Assistant Professor & Head of Department (Department of Chemistry) Shivpati Postgraduate College, Shohratgarh, Siddharth Nagar, Affiliated to Siddharth University, Kapilvastu, Siddharth Nagar, U.P., India e-mail: vksinghsppg666[at]gmail.com Abstract: Green nanotechnology has two goals: producing nanomaterials and products without harming the environment or human health, and producing nano-products that provide solutions to environmental problems. It uses existing principles of green chemistry and green engineering to make nanomaterials and nano-products without toxic ingredients, at low temperatures using less energy and renewable inputs wherever possible, and using lifecycle thinking in all design and engineering stages. 1. Introduction nanofiltration, ultrafiltration membranes. Indeed, among emerging products one can name nanofiber filters, carbon In addition to making nanomaterials and products with less nanotubes and various nanoparticles. Nanotechnology is impact to the environment, green nanotechnology also expected to deal more efficiently with contaminants which means using nanotechnology to make current manufacturing convectional water treatment systems struggle to treat, processes for non-nano materials and products more including bacteria, viruses and heavy metals. This efficiency environmentally friendly. For example, nanoscale generally stems from the very high specific surface area of membranes can help separate desired chemical reaction nanomaterials which increases dissolution, reactivity and products from waste materials from plants. Nanoscale sorption of contaminants. catalysts can make chemical reactions more efficient and less wasteful. Sensors at the nanoscale can form a part Environmental remediation of process control systems, working with nano-enabled Nanoremediation is the use of nanoparticles for information systems. -

A Risk/Benefit Approach to the Application of Iron Nanoparticles for the Remediation of Contaminated Sites in the Environment
A Risk/Benefit Approach to the Application of Iron Nanoparticles A Risk/Benefit Approach to the Application of Iron Nanoparticles for the Remediation of Contaminated Sites in the Environment Paul Bardos, Brian Bone, Daniel Elliott, Niels Hartog, John Henstock, Paul Nathanail Defra Research Project Final Report Defra Project Code: CB0440 Bardos, P., Bone, B., Elliott, D.W., Hartog, N., Henstock, J .E., & Nathanail, C.P. October 2011 1 A Risk/Benefit Approach to the Application of Iron Nanoparticles EXECUTIVE SUMMARY In the context of this report, unless otherwise specified, the term iron nanoparticles (NPs) refer to zero-valent (e.g. Fe(0)) as opposed to any of the oxidised nanoparticles of iron (e.g. Fe(II) or Fe(III) present in one or more forms of oxyhydroxides, carbonates, or other species). In 2004, in a joint report, the Royal Society and Royal Academy of Engineering recommended a precautionary approach to the release of nanomaterials into the environment in the UK, which included a view that the free release of nanomaterials for environmental applications, such as remediation of groundwater, be prohibited until appropriate research has been undertaken to demonstrate that the benefits outweigh the potential risks. Since then, there have been evolutionary rather than revolutionary advances in the understanding of the benefits and potential risks from using iron NPs for the remediation of contaminated soil and groundwater. An increase in field trials, particularly in North America, has been accompanied by an exponential year-on-year increase in peer-reviewed papers on iron NPs and treatment efficacy of various contaminant types but a slower increase in papers studying the potential human health and ecological risks. -
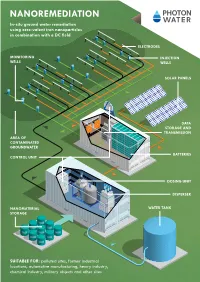
Nanoremediation In-Situ Ground Water Remediation Using Zero-Valent Iron Nanoparticles in Combination with a DC Field
NANOREMEDIATION In-situ ground water remediation using zero-valent iron nanoparticles in combination with a DC field ELECTRODES MONITORING INJECTION WELLS WELLS SOLAR PANELS DATA STORAGE AND TRANSMISSION AREA OF CONTAMINATED GROUNDWATER BATTERIES CONTROL UNIT DOSING UNIT DISPERSER NANOMATERIAL WATER TANK STORAGE SUITABLE FOR: polluted sites, former industrial locations, automotive manufacturing, heavy industry, chemical industry, military objects and other sites ABOUT nanORemedIATION Nanoremediation relies on the use of zero-valent iron Large-scale demonstrations at real contaminated sites DC optimised nanocomposite material nanoparticles (nZVI) in reducing water contaminants. An have confirmed the performance of Nanoremediation, Materials used: (depending on specific parameters with or without surface treatment) additional electric field enhances the transport of nano- especially in low permeability soils. particles in an aquifer environment and improves the Nanocomposite materials containing ZVI nanoparticles are applied in a remediation performance of nZVI as well as lowering At the same time the overall costs are reduced substanti- suspension depending on contaminants, soil type and type of application overall costs substantially. ally thanks to the transfer for chemical reduction from the The nanocomposite is shipped either in a ready-to-use water suspension or in cathodes to contaminants through nZVI and then increa- dry powder form, which can be stored indefinitely The applied electric field both reduces the adhesion force sing its longevity. between nZVI particles and the surfaces of sedimentary rocks and enhances their reactivity and longevity in con- An additional means of reducing costs is the use of less Process: taminated groundwater environments. reactive nanocomposite material of zero-valent iron. 1 Dispersion and dosing 4 Monitoring Two forms of nanomaterials are produced and used for An integral part of the solution is the monitoring of the Why use nanoremediation? site remediation. -

(Nano)Remediation Challenges
European Union | European Regional Development Fund TANIA - Overview of (Nano)remediation Challenges Introduction Document Objectives The present document summarises the findings from exchange among TANIA project partners throughout the first year of work (2017). It is designed as a tool to support further exchange in Project Year 2 (2018). During Year 2, partners and their stakeholders (TANIA Project Stakeholders – TPS) move from activities designed to set the scene for their territories and understand the nanoremediation concept (step 1 of activities), towards interregional and regional exchange to Merge Expertise, thus identifying practical policy solutions to nanoremediation challenges. TANIA partners participate in the project, as they believe that nanoremediation can provide opportunities for their regional remediation activities. They started the project seeking to understand whether or not the following hypothesis can be relevant for their regions. HYPOTHESIS Nanoremediation has the potential to provide significant comparative advantages in relation to current, time-consuming solutions for remediation of polluted soil and water. It is an innovative, low cost technology, with the potential to save time and money in treating several types of contaminants, with minimal risks in terms of production and use. Nanoremediation can provide excellent environmental and economic opportunities, especially through opening of new markets, strengthening of circular economy and creation of new jobs. Should this hypothesis prove correct, partners could design the best way to insert the concept into their selected regional policy instruments. 1 (Nano)remediation During the first year of exchange, project partners specifically requested that the scope of project analysis be widened to cover not only nanoremediation, but also novel techniques for remediation in general.