Correlating Sensory Attributes, Textural Parameters and Volatile Organic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Studies on Genetic Diversity of Japanese and Vietnamese Melon Germplasm by Using Molecular Markers
Studies on genetic diversity of Japanese and Vietnamese melon germplasm by using molecular markers 2019, September Tran Phuong Dung Graduate School of Environmental and Life Science (Doctor’s course) OKAYAMA UNIVERSITY 1 Table of contents Chapter 1. General introduction .................................................................................................................. 3 1.1. Phylogenetic relationships in genus Cucumis .............................................................................. 4 1.2. Intraspecific classification and domestication history of melon ..................................................... 9 1.3. Asia – the origin center of modern melon cultivars ....................................................................... 16 Chapter 2. Genetic diversity of Japanese melon breeding lines ............................................................... 18 2.1. Introduction ..................................................................................................................................... 18 2.2. Materials and Methods ................................................................................................................... 19 2.3. Result ............................................................................................................................................... 23 2.4. Discussion ........................................................................................................................................ 28 Chapter 3. Development of RAPD‐derived STS -

High Tunnel Melon and Watermelon Production
High Tunnel Melon and Watermelon Production University of Missouri Extension M173 Contents Author Botany 1 Lewis W. Jett, Division of Plant Sciences, University of Missouri-Columbia Cultivar selection 3 Editorial staff Transplant production 4 MU Extension and Agricultural Information Planting in the high tunnel 5 Dale Langford, editor Dennis Murphy, illustrator Row covers 6 On the World Wide Web Soil management and fertilization 6 Find this and other MU Extension publications on the Irrigation 7 Web at http://muextension.missouri.edu Pollination 7 Photographs Pruning 8 Except where noted, photographs are by Lewis W. Jett. Trellising 8 Harvest and yield 9 Marketing 10 Pest management 10 Useful references 14 Melon and watermelon seed sources 15 Sources of high tunnels (hoophouses) 16 For further information, address questions to College of Dr. Lewis W. Jett Agriculture Extension State Vegetable Crops Specialist Food and Natural Division of Plant Sciences Resources University of Missouri Columbia, MO 65211 Copyright 2006 by the University of Missouri Board of Curators E-mail: [email protected] College of Agriculture, Food and Natural Resources High Tunnel Melon and Watermelon Production igh tunnels are low-cost, passive, melo has several botanical subgroups (Table 1). solar greenhouses that use no fossil In the United States, reticulatus and inodorus are Hfuels for heating or venting (Figure commercially grown, while the remaining groups 1). High tunnels can provide many benefits to are grown for niche or local markets. horticulture crop producers: The cantaloupe fruit that most Americans • High tunnels are used to lengthen the are familiar with is not actually a true cantaloupe. -

Generate Evaluation Form
Great Lakes Fruit, Vegetable & Farm Market EXPO Michigan Greenhouse Growers EXPO December 5-7, 2017 DeVos Place Convention Center, Grand Rapids, MI Vine Crops Where: Grand Gallery (main level) Room E & F MI Recertification credits: 2 (1B, COMM CORE, PRIV CORE) OH Recertification credits: 1 (presentations as marked) CCA Credits: PM(1.0) CM(1.0) Moderator: Ben Phillips, Vegetable Extension Educator, MSU Extension, Saginaw, MI 9:00 am Integrated Management of Powdery Mildew and Gummy Stem Blight on Cucurbits: A Florida Perspective (OH: 2B, 0.5 hr) Gary Vallad, Plant Pathology Dept., Univ. of Florida 9:30 am Perspectives and Opportunities for Growing Orange Flesh and Specialty Melons Jonathan Schultheis, Dept. Horticultural Science, North Carolina State University 10:00 am Best Management Practices: Controlling Insects While Protecting Pollinators (OH: CORE, 0.5 hr) Rick Foster, Entomology Dept., Purdue Univ. 10:30 am Cover Cropping in Melons for Reduced Washing After Harvest Chris Gunter, Horticultural Science Dept., North Carolina State Univ. 11:00 am Session Ends Perspectives and Opportunities for Growing Orange Flesh and Specialty Melons Jonathan R. Schultheis North Carolina State University Department of Horticultural Science 2721 Founders Drive, 264 Kilgore Raleigh, NC 27695-7609 [email protected] Orange Flesh Melon Types: The landscape of orange flesh muskmelons (cantaloupes) has changed dramatically over the past five to ten years. Traditionally, there were two primary orange flesh melon types grown and sold; western types, which are fruits that range in size from about 3 to 5 pounds which are heavily netted and not sutured, and eastern types which have some netting, have a shorter shelf life but have a larger fruit size than a western melon, and tend to have a softer flesh with apparent more flavor than a western melon. -

Histological Study of Organogenesis in Cucumis Melo L. After Genetic Transformation: Why Is It Difficult to Obtain Transgenic Plants? V Chovelon, V
Histological study of organogenesis in Cucumis melo L. after genetic transformation: why is it difficult to obtain transgenic plants? V Chovelon, V. Restier, N. Giovinazzo, Catherine Dogimont, J. Aarouf To cite this version: V Chovelon, V. Restier, N. Giovinazzo, Catherine Dogimont, J. Aarouf. Histological study of organo- genesis in Cucumis melo L. after genetic transformation: why is it difficult to obtain transgenic plants?. Plant Cell Reports, Springer Verlag, 2011, 30 (11), pp.2001-2011. 10.1007/s00299-011-1108-9. hal- 01332269 HAL Id: hal-01332269 https://hal.archives-ouvertes.fr/hal-01332269 Submitted on 29 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial| 4.0 International License Version définitive du manuscrit publié dans / Final version of the manuscript published in : Plant Cell Reports, 2011, vol 30 (11) :2001-2011 DOI: 10.1007/s00299-011-1108-9 Histological Study of Organogenesis in Cucumis melo L. after genetic transformation: why is it difficult to obtain transgenic plants? V. Chovelon .V. Restier . N. Giovinazzo . C. Dogimont J. Aarrouf INRA Avignon, UR1052, Unité de Génétique et d’Amélioration des Fruits et Légumes, BP 94, 84143 Montfavet Cedex, France e-mail : [email protected] J. -

Transformation of 'Galia' Melon to Improve Fruit
TRANSFORMATION OF ‘GALIA’ MELON TO IMPROVE FRUIT QUALITY By HECTOR GORDON NUÑEZ-PALENIUS A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2005 Copyright 2005 by Hector Gordon Nuñez-Palenius This document is dedicated to the seven reasons in my life, who make me wake up early all mornings, work hard in order to achieve my objectives, dream on new horizons and goals, feel the beaty of the wind, rain and sunset, but mostly because they make me believe in God: my Dads Jose Nuñez Vargas and Salvador Federico Nuñez Palenius, my Moms Janette Ann Palenius Alberi and Consuelo Nuñez Solís, my wife Nélida Contreras Sánchez, my son Hector Manuel Nuñez Contreras and my daughter Consuelo Janette Nuñez Contreras. ACKNOWLEDGMENTS This dissertation could not have been completed without the support and help of many people who are gratefully acknowledged here. My greatest debt is to Dr. Daniel James Cantliffe, who has been a dedicated advisor and mentor, but mostly an excellent friend. He provided constant and efficient guidance to my academic work and research projects. This dissertation goal would not be possible without his insightful, invariable and constructive criticism. I thank Dr. Daniel J. Cantliffe for his exceptional course on Advanced Vegetable Production Techniques (HOS-5565) and the economic support for living expenses during my graduate education in UF. I extend my appreciation to my supervisory committee, Dr. Donald J. Huber, Dr. Harry J. Klee, and Dr. Donald Hopkins, for their academic guidance. -
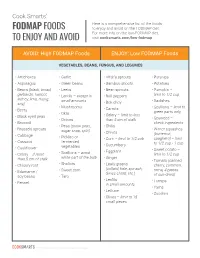
Fodmapfoods to Enjoy and Avoid
Cook Smarts’ Here is a comprehensive list of the foods FODMAP FOODS to enjoy and avoid on the FODMAP diet. For more info on the low-FODMAP diet, TO ENJOY AND AVOID visit cooksmarts.com/low-fodmap AVOID: High FODMAP Foods ENJOY: Low FODMAP Foods VEGETABLES, BEANS, FUNGUS, AND LEGUMES • Artichokes • Garlic • Alfalfa sprouts • Parsnips • Asparagus • Green beans • Bamboo shoots • Potatoes • Beans (black, broad, • Leeks • Bean sprouts • Pumpkin – garbanzo, haricot, • Lentils – except in • Bell peppers limit to 1/2 cup kidney, lima, mung, small amounts • Radishes soy) • Bok choy • Mushrooms • Scallions – limit to • Beets • Carrots • Okra green parts only • Black-eyed peas • Celery – limit to less • Onions than 5 cm of stalk • Seaweed – • Broccoli check ingredients • Peas (snow peas, • Chilis • Brussels sprouts • Winter squashes sugar snap, split) • Chives • Cabbage (butternut, • Pickled or • Corn – limit to 1/2 cob spaghetti) – limit • Cassava fermented • Cucumbers to 1/2 cup - 1 cup • Cauliflower vegetables • Eggplant • Sweet potato – • Celery – if more • Scallions – avoid limit to 1/2 cup white part of the bulb • Ginger than 5 cm of stalk • Tomato (canned, • Chicory root • Shallots • Leafy greens cherry, common, • Sweet corn (collard, kale, spinach, roma, 4 pieces • Edamame / Swiss chard, etc.) soy beans • Taro of sun-dried) • Lentils – • Turnips • Fennel in small amounts • Yams • Lettuce • Zucchini • Olives – limit to 15 small pieces © 2018 Cook Smarts | All Rights Reserved | Page 1 AVOID: High FODMAP Foods ENJOY: Low FODMAP Foods FRUITS -

02001554.Pdf
Preliminary characterization of germplasm collected in Apulia and Albania Ricciardi L., Filippetti A. in Ricciardi L. (ed.), Myrta A. (ed.), De Castro F. (ed.). Italo-Albanian cooperation for the enhancement of plant biodiversity Bari : CIHEAM Options Méditerranéennes : Série A. Séminaires Méditerranéens; n. 47 2001 pages 151-169 Article available on line / Article disponible en ligne à l’adresse : -------------------------------------------------------------------------------------------------------------------------------------------------------------------------- http://om.ciheam.org/article.php?IDPDF=2001554 -------------------------------------------------------------------------------------------------------------------------------------------------------------------------- To cite this article / Pour citer cet article -------------------------------------------------------------------------------------------------------------------------------------------------------------------------- Ricciardi L., Filippetti A. Preliminary characterization of germplasm collected in Apulia and Albania. In : Ricciardi L. (ed.), Myrta A. (ed.), De Castro F. (ed.). Italo-Albanian cooperation for the enhancement of plant biodiversity. Bari : CIHEAM, 2001. p. 151-169 (Options Méditerranéennes : Série A. Séminaires Méditerranéens; n. 47) -------------------------------------------------------------------------------------------------------------------------------------------------------------------------- http://www.ciheam.org/ http://om.ciheam.org/ -

Melon Match up Match the Melon Name and Description with Its Picture
MELON MADNESS! Vine growing fruit, melons are members of the Cucurbitaceous family, with their next closest relatives being squashes and cucumbers. Ancient Egyptians have been cultivating melons 2000 years BC, and were introduced to America by the Spanish settlers during the 15th and 16th century. There are numerous varieties of melons that differ in size, color and sweetness of their fruit. CLASSROOM DISCUSSION/ACTIVITY Melon Match Up Match the melon name and description with its picture. A B C D E 1. Gac Melon 2. 3. Horned Melon 4. Canary 5. Cantaloupe Gac is primarily a The most popular Watermelon It gets its name from Melon Southeast Asian melon in the United Containing 92% the horn-like spikes Named after the fruit. It is not very States, this melon water, the and has a texture that bright yellow abundant as it has a was first cultivated watermelon is is very similar to that of color of the short harvet season. in the 1700’s in the appropriately a cucumber. The taste canary bird, the The seeds of the fruit Italian village of names. The is tart-like, and often canary melon has are rich in flavor and Cantalup. largest recorded described as a a prominent are cooked with rice Cantaloupe is a rich watermelon combination of sweet flavor and in Vietnam. Its juice source for dietary weighed 262 cucumber and zucchini. tastes like is becoming popular fiber, vitamin A, pounds. cantaloupe. out side of Asia, as it vitamin C, iron, is very high in phosphorus and nutrients. 1. _____ 2.______ 3.______ 4.______ 5.______ potassium. -

Alginate-Based Edible Coating to Enhance Quality And
ALGINATE-BASED EDIBLE COATING TO ENHANCE QUALITY AND EXTEND SHELF LIFE OF FRESH-CUT WATERMELON (CITRULLUS LANATUS) A Thesis by RABIA ESMA SIPAHI Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE August 2012 Biological and Agricultural Engineering Alginate-based Edible Coating to Enhance Quality and Extend Shelf Life of Fresh- Cut Watermelon (Citrullus Lanatus) Copyright 2012 Rabia Esma Sipahi ALGINATE-BASED EDIBLE COATING TO ENHANCE QUALITY AND EXTEND SHELF LIFE OF FRESH-CUT WATERMELON (CITRULLUS LANATUS) A Thesis by RABIA ESMA SIPAHI Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Approved by: Chair of Committee, Elena Castell-Perez Committee Members, Rosana Moreira Carmen Gomes Alejandro Castillo Head of Department, Stephen W. Searcy August 2012 Biological and Agricultural Engineering iii ABSTRACT Alginate-based Edible Coating to Enhance Quality and Extend Shelf life of Fresh-Cut Watermelon (Citrullus Lanatus). (August 2012) Rabia Esma Sipahi, B.S., Ankara University Chair of Advisory Committee: Dr. Elena Castell-Perez Fresh-cut watermelon is appreciated for its taste, flavor, and juiciness. However, there are challenges in maintaining the freshness since fresh-cut processing of fruits promotes faster deterioration. Our objective was to determine the effectiveness of multilayered antimicrobial edible coating on the shelf-life of fresh-cut watermelon while keeping its original attributes for longer, without affecting its sensory properties. A set of solutions containing sodium alginate (0.5, 1, 2% w/w), beta- cyclodextrin, trans-cinnamaldehyde (antimicrobial agent), pectin, and calcium lactate were used as coating systems for fresh-cut watermelon cylinders. -
Melon & Watermelon
Melon & Watermelon Assortment 2019-2020 Meet our Melon & Watermelon team Dear customer, We are happy to present to you our new international melon booklet: an overview of our melon and watermelon varieties. Our product development activities in recent years have led us into new production areas and consumer markets. Hence, our range today includes varieties that are grown worldwide. Nevertheless, our international focus does not exclude new labels for local markets like Asian Jewels, a range of melon types and varieties specially bred for the various Asian markets. There are different ways of growing melons - e.g. open field or indoor, horizontal or vertical, local-for-local or further afield because of climate influences - but, ultimately, we all share the same goal: satisfied consumers − anywhere, anytime. Should you have any questions about this booklet, please feel free to contact your local Rijk Zwaan representative. Crop Team Melon & Watermelon Rijk Zwaan Crop coordinator Maarten den Hertog [email protected] Crop coordinator Diego Maestre Claramunt [email protected] Crop coordinator Vincent van Wolferen [email protected] 2 Americas Argentina Ignacio Damián Lopez [email protected] Brazil (melon) Weber Carlos Pinheiro [email protected] Brazil (watermelon) Eric Rodrigo Bezerra [email protected] Central America Darwin Lopez Acevedo [email protected] Chile Carlos Hurel Estay [email protected] Mexico Lionel Aragon Garcia [email protected] USA Joe Vandiver [email protected] USA -

Biodiversity in Vegetable Crops, a Heritage to Save: the Case of Puglia Region
Italian Journal of Agronomy 2013; volume 8:e4 Biodiversity in vegetable crops, a heritage to save: the case of Puglia region Antonio Elia,1 Pietro Santamaria2 1Department of the Science of Agriculture, Food and Environment (SAFE), University of Foggia; 2Department of Agricultural and Environmental Science, University of Bari Aldo Moro, Italy among the leading regions for the productions of broccoli raab, celery, Abstract parsley, processing tomato, artichoke, endive and escarole, cabbage, fennel, lettuce, cucumber, cauliflower and broccoli, early potato, and The biodiversity in vegetable crops is composed by the genetic diver- asparagus (all with more than 20% of the national area). The region is sity, as species diversity (interspecific diversity) and as diversity of particularly rich in local vegetable varieties, obtained by farmers them- genes within a species (intraspecific diversity) referring to the veg- selves after repeated simple selection procedures generation after etable grown varieties, and by the diversity of agro-ecosystems (agro- generation. The local varieties for which there is a strong link with the biodiversity). Intraspecific diversity is very ample in vegetable crops Puglia traditions and which are described in this review are: carota di and is not reflected, at least not to the same extent, in other groups of Polignano (Polignano carrot) and carota di sant’Ippazio (Saint Ippazio crops. The labour operated by farmers over centuries of selection has carrot) (Apiaceae), cipolla di Acquaviva delle Fonti (Acquaviva delle led to the creation of a plurality of local varieties, following domestica- Fonti onion) and cipolla bianca di Margherita (Margherita white tion of cultivated forms, and wide agro-biodiversity, a precious her- onion) (Liliaceae), cima di rapa (broccoli raab) (Brassicaceae), unripe itage both from a genetic and a cultural-historical point of view. -

PRODUCE SUBSTITUTION GUIDE • 1 Cup (250 Ml) Raspberries Or Strawberries (Fresh)
PRODUCE SUBSTITUTION GUIDE • 1 cup (250 mL) raspberries or strawberries (fresh) Red Apples Substitute 1 lb (500 g) apples with: • 1 lb (500 g) pears (generally softer flesh) • 1 lb (500 g) Asian pears (generally crisper and juicier texture; tart flavor) • 1 lb (500 g) quinces (best for cooking; tart flavor) Cantaloupe Substitute 1 cup (250 mL) chopped cantaloupe with: • 1 cup (250 mL) chopped Persian melon • 1 cup (250 mL) chopped Crenshaw melon • 1 cup (250 mL) chopped Santa Claus melon • 1 cup (250 mL) chopped honeydew melon • 1 cup (250 mL) chopped casaba melon Banana Substitute 1 lb (500 g) ripe Cavendish bananas with: • 1 lb (500 g) very ripe black plantains (larger; less sweet; more starchy; best for baking, mashing, Grapefruit Substitute 1 cup (250 mL) grapefruit segments with: • 1⁄2 cup (125 mL) pummelo segments + 1⁄2 cup (125 mL) and frying) orange segments • 1 cup (250 mL) Ugli fruit™ segments Blackberry Substitute 1 cup (250 mL) blackberries with: • 1 cup (250 mL) tangelo segments • 1 cup (250 mL) loganberries (red color) • 1 cup (250 mL) boysenberries (slightly sweeter) • 1 cup (250 mL) olallieberries (larger; sweeter) Grapes Substitute 1 cup (250 mL) green table grapes with: • 1 cup (250 mL) raspberries (smaller; sweeter) • 1 cup (250 mL) blueberries (purple color; blueberry flavor) Blueberry Substitute 1 cup (250 mL) blueberries with: Honeydew Substitute 1 cup (250 mL) chopped honeydew melon with: • 1 cup (250 mL) huckleberries (more tart) • 1 cup (250 mL) chopped Ogen melon • 1 cup (250 mL) red currants (red color; more