Attractiveness of the Dark Central Floret in Wild Carrots: Do Umbel Size and Height Matter?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Inheritance of Apospory in <Emphasis Type="Italic">
THE INHER,ITANCE OF APOSPOR,¥ IN SCOLOPENDRI UM V ULGARE BY I. ANDEI~SSON-KOTT0 A~D A. E. C4AIICDNEI% Joh~t Innes Horticultu~'al Institution, Me,rton, Londo'Jb (With Plates VI-XII and Two Folders) TIt~ literature on apomixis and apospory is very extensive. Morpho- logical and taxonomic studies, and lately cytological and genetical work, have brought to light various important facts which have not only made possible extensive conclusions but have given rise to speculations in various directions. Among the more general questions which have arisen are those relating to the cause of apomixis and apospory, the relation of polyploidy to apomixis, the relation of polymorphism of plant genera to apomixis and apospory, or generally the part played by apomixis in evolution and species formation, and the alternation of generations. It is impossible here to go into the extensive literature on the subject, it will only be necessary to refer to the recent handbooks by l%osenberg (1930) and Schnarf (1929). It may be mentioned that, in addition to speculations on hybridism in itself as the cause of apomixis, whether on cytological (Winge, 1917) or comparative (Ernst, 1918) grounds, more definite views were expressed on t]zis subject by I~osenberg (1917), Whlkler (1920) and more especially by Hohngren (1919), and by l%osen- berg in his later work (1930); these authors were conscious of the possi- bility of a genetic factor for tendency to apomixis, and have expressed themselves to this effect. More recently Schnarf (1929) expresses himself in the following way: "Die Anlage zur kpomixis diirfte in vielen Fgllen schon vet der Kreuzung vorhanden gewesen sein, and diese dr'trite eher eine ausl6sende als eine unmittelbar hervorrufende gewesen sein." (J-ustafsson (1932) is more definite and considers in more detail the possibilities of the origin of new forms and of apomi~s as a dominant or recessive character in triploid Yfaraxaca and Ar&ie,ra~ia. -

Reproductive Ecology of Heracleum Mantegazzianum
4 Reproductive Ecology of Heracleum mantegazzianum IRENA PERGLOVÁ,1 JAN PERGL1 AND PETR PYS˘EK1,2 1Institute of Botany of the Academy of Sciences of the Czech Republic, Pru˚honice, Czech Republic; 2Charles University, Praha, Czech Republic Botanical creature stirs, seeking revenge (Genesis, 1971) Introduction Reproduction is the most important event in a plant’s life cycle (Crawley, 1997). This is especially true for monocarpic plants, which reproduce only once in their lifetime, as is the case of Heracleum mantegazzianum Sommier & Levier. This species reproduces only by seed; reproduction by vegetative means has never been observed. As in other Apiaceae, H. mantegazzianum has unspecialized flowers, which are promiscuously pollinated by unspecialized pollinators. Many small, closely spaced flowers with exposed nectar make each insect visitor to the inflorescence a potential and probable pollinator (Bell, 1971). A list of insect taxa sampled on H. mantegazzianum (Grace and Nelson, 1981) shows that Coleoptera, Diptera, Hemiptera and Hymenoptera are the most frequent visitors. Heracleum mantegazzianum has an andromonoecious sex habit, as has almost half of British Apiaceae (Lovett-Doust and Lovett-Doust, 1982); together with perfect (hermaphrodite) flowers, umbels bear a variable propor- tion of male (staminate) flowers. The species is considered to be self-compati- ble, which is a typical feature of Apiaceae (Bell, 1971), and protandrous (Grace and Nelson, 1981; Perglová et al., 2006). Protandry is a temporal sep- aration of male and female flowering phases, when stigmas become receptive after the dehiscence of anthers. It is common in umbellifers. Where dichogamy is known, 40% of umbellifers are usually protandrous, compared to only about 11% of all dicotyledons (Lovett-Doust and Lovett-Doust, 1982). -

Invasive Weeds of the Appalachian Region
$10 $10 PB1785 PB1785 Invasive Weeds Invasive Weeds of the of the Appalachian Appalachian Region Region i TABLE OF CONTENTS Acknowledgments……………………………………...i How to use this guide…………………………………ii IPM decision aid………………………………………..1 Invasive weeds Grasses …………………………………………..5 Broadleaves…………………………………….18 Vines………………………………………………35 Shrubs/trees……………………………………48 Parasitic plants………………………………..70 Herbicide chart………………………………………….72 Bibliography……………………………………………..73 Index………………………………………………………..76 AUTHORS Rebecca M. Koepke-Hill, Extension Assistant, The University of Tennessee Gregory R. Armel, Assistant Professor, Extension Specialist for Invasive Weeds, The University of Tennessee Robert J. Richardson, Assistant Professor and Extension Weed Specialist, North Caro- lina State University G. Neil Rhodes, Jr., Professor and Extension Weed Specialist, The University of Ten- nessee ACKNOWLEDGEMENTS The authors would like to thank all the individuals and organizations who have contributed their time, advice, financial support, and photos to the crea- tion of this guide. We would like to specifically thank the USDA, CSREES, and The Southern Region IPM Center for their extensive support of this pro- ject. COVER PHOTO CREDITS ii 1. Wavyleaf basketgrass - Geoffery Mason 2. Bamboo - Shawn Askew 3. Giant hogweed - Antonio DiTommaso 4. Japanese barberry - Leslie Merhoff 5. Mimosa - Becky Koepke-Hill 6. Periwinkle - Dan Tenaglia 7. Porcelainberry - Randy Prostak 8. Cogongrass - James Miller 9. Kudzu - Shawn Askew Photo credit note: Numbers in parenthesis following photo captions refer to the num- bered photographer list on the back cover. HOW TO USE THIS GUIDE Tabs: Blank tabs can be found at the top of each page. These can be custom- ized with pen or marker to best suit your method of organization. Examples: Infestation present On bordering land No concern Uncontrolled Treatment initiated Controlled Large infestation Medium infestation Small infestation Control Methods: Each mechanical control method is represented by an icon. -

Ornamental Garden Plants of the Guianas Pt. 2
Surinam (Pulle, 1906). 8. Gliricidia Kunth & Endlicher Unarmed, deciduous trees and shrubs. Leaves alternate, petiolate, odd-pinnate, 1- pinnate. Inflorescence an axillary, many-flowered raceme. Flowers papilionaceous; sepals united in a cupuliform, weakly 5-toothed tube; standard petal reflexed; keel incurved, the petals united. Stamens 10; 9 united by the filaments in a tube, 1 free. Fruit dehiscent, flat, narrow; seeds numerous. 1. Gliricidia sepium (Jacquin) Kunth ex Grisebach, Abhandlungen der Akademie der Wissenschaften, Gottingen 7: 52 (1857). MADRE DE CACAO (Surinam); ACACIA DES ANTILLES (French Guiana). Tree to 9 m; branches hairy when young; poisonous. Leaves with 4-8 pairs of leaflets; leaflets elliptical, acuminate, often dark-spotted or -blotched beneath, to 7 x 3 (-4) cm. Inflorescence to 15 cm. Petals pale purplish-pink, c.1.2 cm; standard petal marked with yellow from middle to base. Fruit narrowly oblong, somewhat woody, to 15 x 1.2 cm; seeds up to 11 per fruit. Range: Mexico to South America. Grown as an ornamental in the Botanic Gardens, Georgetown, Guyana (Index Seminum, 1982) and in French Guiana (de Granville, 1985). Grown as a shade tree in Surinam (Ostendorf, 1962). In tropical America this species is often interplanted with coffee and cacao trees to shade them; it is recommended for intensified utilization as a fuelwood for the humid tropics (National Academy of Sciences, 1980; Little, 1983). 9. Pterocarpus Jacquin Unarmed, nearly evergreen trees, sometimes lianas. Leaves alternate, petiolate, odd- pinnate, 1-pinnate; leaflets alternate. Inflorescence an axillary or terminal panicle or raceme. Flowers papilionaceous; sepals united in an unequally 5-toothed tube; standard and wing petals crisped (wavy); keel petals free or nearly so. -
Native Plants North Georgia
Native Plants of North Georgia A photo guide for plant enthusiasts Mickey P. Cummings · The University of Georgia® · College of Agricultural and Environmental Sciences · Cooperative Extension CONTENTS Plants in this guide are arranged by bloom time, and are listed alphabetically within each bloom period. Introduction ................................................................................3 Blood Root .........................................................................5 Common Cinquefoil ...........................................................5 Robin’s-Plantain ..................................................................6 Spring Beauty .....................................................................6 Star Chickweed ..................................................................7 Toothwort ..........................................................................7 Early AprilEarly Trout Lily .............................................................................8 Blue Cohosh .......................................................................9 Carolina Silverbell ...............................................................9 Common Blue Violet .........................................................10 Doll’s Eye, White Baneberry ...............................................10 Dutchman’s Breeches ........................................................11 Dwarf Crested Iris .............................................................11 False Solomon’s Seal .........................................................12 -

Milkweed Identification Guide
Milkweeds of the Santa Rosa and San Jacinto Mountains National Monument True Milkweeds Climbing Milkweeds 1. California Milkweed 8. Fringed Twinevine 2. Desert Milkweed 9. Hairy/Rambling Vine 3. Narrowleaf Milkweed Milkweed 4. Rush Milkweed 10. Utah Vine Milkweed 5. Tropical Milkweed 11. Wavy Leaf Twinevine 6. White-stemmed Milkweed 7. Woollypod Milkweed Apps for Milkweed Identification: South California Wildflowers by Wildflower Search Identification information provided by MonarchWatch.org and Calscape.org California Milkweed (Asclepias californica) • Flower: Umbels are pendulous. 10 +/- flowers per umbel. Color varies from a dull or bright pink to lavender. Wool like hair is everywhere, except on the flower head, but there is hair on the outside of the corolla lobe. No horns present. Corolla reflexes backward. Flower head is 1/3 – 1/2 in (8-10 mm) long. • Foliage: Thick stems and leaves are covered with dense hair. Stands erect to reclining. Leaf arrangement is opposite and attachment is sessile or has a short petiole. Mature plants have multiple stems. • Habitat: Flats, grassy slopes, and open woods. • Height: 36-48 in (91 ½ – 122 cm). Can grow in clumps up to 42 inches in width. • Leaves: Ovate, oblong to lanceolate. Approximately 2-7 in (5-18 cm) long and 1-3 in (2 ½ – 7 ½ cm) wide. Desert Milkweed (Asclepias erosa) • Flower: Color is white to yellow. Horns protrude from the hoods. The corolla folds back from the hoods after blossoming. Umbels stand erect with 20 +/- flowers. Thick peduncle. Flower is 1/8 in (5-6 mm) long. • Foliage: Color is dull if leaves are covered with a fine cream-colored hair, but can also be glabrous. -
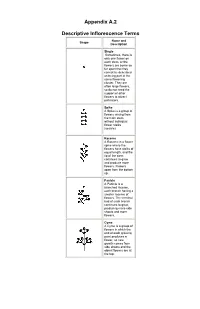
Appendix A.2 Descriptive Inflorescence Terms
Appendix A.2 Descriptive Inflorescence Terms Name and Shape Description Single Sometimes, there is only one flower on each stem, or the flowers are borne so far apart that they cannot be described as being part of the same flowering cluster. They are often large flowers, so do not need the support of other flowers to attract pollinators. Spike A Spike is a group of flowers arising from the main stem, without individual flower stalks (sessile). Raceme A Raceme is a flower spike where the flowers have stalks of equal length, and the tip of the stem continues to grow and produce more flowers. Flowers open from the bottom up. Panicle A Panicle is a branched raceme, each branch having a smaller raceme of flowers. The terminal bud of each branch continues to grow, producing more side shoots and more flowers. Cyme A Cyme is a group of flowers in which the end of each growing point produces a flower, so new growth comes from side shoots and the oldest flowers are at the top. Verticillaster A Verticillaster is a whorled inflorescence, where the flowers are borne in rings at intervals up the stem. The tip continues to grow, producing more whorls. This type of inflorescence is common in members of the Deadnettle/Mint Family (Lamiaceae). Corymb A Corymb is a flower cluster where all the flowers are at the same level, with flower stalks of different lengths, forming a flat-topped flower cluster. Umbel An Umbel is a flower head in which all the flower stalks are of the same length, so that the flower head is rounded like an umbrella. -

Excentradenia, a New Genus of Malpighiaceae from South America
Contr. Univ. Michisan Herb. 2I:29-36. 1997. EXCENTRADENIA, A NEW GENUS OF MALPIGHIACEAE FROM SOUTH AMERICA William R. Anderson University of Michigan Herbarium North University Building Ann Arbor. Michisan 48109-1057 When I describedHiraea propinqu.ain 1994| discussedthe problems created rn Hiraea by the inclusion of H. adenophora Sandwith and its relatives,but hesi- tated to segregatethat complex as a distinct genus (Anderson 1994,pp. I32-I33). Now I have come to the conclusionthat segregationis inevitable and desirable,so that Hiraea can return to the homogeneity that distinguished it before these spe- cieswere described. Excentradenia W. R. Anderson, gen. nov.-Tvpp.: Excentradeniaatlenophora (Sand- with) W. R. Anderson. Lianae lignosae. Lamina foliorum eglandulosavel margine glandulis parvis instructa, venis tertiariis scalariformibus;petiolus biglandulosus;stipulae parvae, triangulares,basi petioli vel caule juxta petiolum portatae, vel nullae. Inflorescen- tia axillaris racemus3-7 (-9) umbellarum 4-florarum; bracteaefloriferae eglandu- losae;pedunculus brevis vel nullus; 1 bracteola cujusqueparis eglandulosa,altera uniglandulifera glandulo versus centrum umbellae excentrico; pedicellus in ala- bastro circinatus. Sepala triangularia vel ovata, apice acuta revolutaque. Petala lutea, glabra. Stamina 10, omnia fertilia. Styli 3, stigmate introrso, apice dorsaliter truncati, apiculati, vel breviuncinati. Samaraeala lateralis membranacea,plerum- que subcircularis.apice usque ad nucem incisa, basi continua vel interdum usque ad nucem incisa,nuce -

EXTENSION EC1257 Garden Terms: Reproductive Plant Morphology — Black/PMS 186 Seeds, Flowers, and Fruitsextension
4 color EXTENSION EC1257 Garden Terms: Reproductive Plant Morphology — Black/PMS 186 Seeds, Flowers, and FruitsEXTENSION Anne Streich, Horticulture Educator Seeds Seed Formation Seeds are a plant reproductive structure, containing a Pollination is the transfer of pollen from an anther to a fertilized embryo in an arrestedBlack state of development, stigma. This may occur by wind or by pollinators. surrounded by a hard outer covering. They vary greatly Cross pollinated plants are fertilized with pollen in color, shape, size, and texture (Figure 1). Seeds are EXTENSION from other plants. dispersed by a variety of methods including animals, wind, and natural characteristics (puffball of dandelion, Self-pollinated plants are fertilized with pollen wings of maples, etc.). from their own fl owers. Fertilization is the union of the (male) sperm nucleus from the pollen grain and the (female) egg nucleus found in the ovary. If fertilization is successful, the ovule will develop into a seed and the ovary will develop into a fruit. Seed Characteristics Seed coats are the hard outer covering of seeds. They protect seed from diseases, insects and unfavorable environmental conditions. Water must be allowed through the seed coat for germination to occur. Endosperm is a food storage tissue found in seeds. It can be made up of proteins, carbohydrates, or fats. Embryos are immature plants in an arrested state of development. They will begin growth when Figure 1. A seed is a small embryonic plant enclosed in a environmental conditions are favorable. covering called the seed coat. Seeds vary in color, shape, size, and texture. Germination is the process in which seeds begin to grow. -

Plant Anatomy & Plant Parts
Flower and Inflorescence Types What you need to know to identify plants Wildland Plant Identification REM 252 Flowers and Reproduction New Plant Flower Fruit/Seed Pollination Structure of Flowers Structure of Flowers PERFECT Flower = includes stamens (♂ or male) AND pistil (♀ or female) IMPERFECT Flower = has stamens (♂ or male) OR pistil (♀ or female) but not both Perfect Flowers Imperfect Flowers Monoecious - ♂ and ♀ flowers on SAME plant Dioecious - ♂ and ♀ flowers on SEPARATE plants Monoecious Dioecious Male Male Plant Female Male Female Female Plant Imperfect Flowers Monoecious - ♂ and ♀ flowers on SAME plant Dioecious - ♂ and ♀ flowers on SEPARATE plants Monoecious Dioecious Male Plant Male Female Female Plant Structure of Flowers Flower Inflorescence Structure of Flowers Inflorescence - Group of flowers Structure of Flowers Catkin = a dense spike or raceme with many small, usually naked, flowers. Cyme = a convex or flat-topped flower cluster with the central flower the first to open. A determinate inflorescence. Panicle Flowers attached to branching branches Raceme Flowers attached to main stalk with pedicels Spike Flowers connected directly to main stalk (not on panicle branches off main stem) * You do not need to memorize the terms pedicel or sessile Umbel All pedicles appx. same length connect to stalk at same location This is a compound umbel (umbel with umbels at the tips) Corymb Raceme with longer (uneven) pedicles at bottom of inflorescence Flowers appear to be at the same height Corymb = a simple racemose inflorescence that is flat-topped. An indeterminate inflorescence. Cyme Oldest flower in center. Younger flowers grow on outside with longer pedicles so flowers appear to be at the same height. -

Contributions from the United States National Herbarium [Electronic
LEIBERGIA, A NEW GENUS OF UMBELL1FEILE FROM THE COLUMBIA RIVER REGION. ISy John M. Coci/tkr and .1. N. Kose. part A of the material upon which this new genus is based has been in the National Herbarium a half dozen years, and is doubtless to be found in many of our herbaria, either undetermined or incorrectly named. The receipt of some excellent additional material from Mr. W. N. Suksdorf and an abundant supply from one of our field agents Mr. John U. Leiberg, gives the opportunity of separating what has appeared to us for some time to be a new genus. Leibergia, gen. nov. , Calyx teeth obsolete. Fruit flattened laterally, linear, beaked, glabrous; stylo- podium wanting. Carpels only slightly flattened dorsalJy, with five filiform ribs, (he two lateral a little more prominent and turned inward; oil tubes small, single in the intervals, two on the commissural side. Seed face broad, slightly concave, but when dry becoming more or less involute. Slender, glabrous, acaulescent plants from small globose tubers: leaves ternately divided intolong, filiform leaflets; umbels irregular; fruit subsessile; flowers white. The affinities of this genus are doubtful. It has much the habit of the bulbous Peucedanums of the Northwest, but the lateral flattening of the fruit and the absence of wings readily exclude it from that genus. Our plant is very closely allied to the two anomalous species, Pcucedanum ambigumn an.l P. leptocarpitm, which likewise have linear fruit with very narrow lateral wings, and is, perhaps, congeneric with them. The dorsal flattening of the fruit has hitherto been considered s"ich an important character that it seems best, at least for the present, to base our generic distinction on this difference rather than to include these two species in the new genus. -

Emergent Flowering Plants, A-C – Pg.1
Starflower Image Herbarium & Landscaping Pages Emergent Flowering Plants, A-C – pg.1 Starflower Image Herbarium Emergent Flowering Plants, A-C © Starflower Foundation, 1996-2007 Washington Native Plant Society These species pages has been valuable and loved for over a decade by WNPS members and the PNW plant community. Untouched since 2007, these pages have been archived for your reference. They contain valuable identifiable traits, landscaping information, and ethnobotanical uses. Species names and data will not be updated. To view updated taxonomical information, visit the UW Burke Herbarium Image Collection website at http://biology.burke.washington.edu/herbarium/imagecollection.php. For other useful plant information, visit the Native Plants Directory at www.wnps.org. Compiled September 1, 2018 Starflower Image Herbarium & Landscaping Pages Emergent Flowering Plants, A-C – pg.2 Contents Achillea millefolium ................................................................................................................................................................ 4 Yarrow ................................................................................................................................................................................. 4 Achlys triphylla ....................................................................................................................................................................... 6 Vanilla Leaf .........................................................................................................................................................................