Molecular Phylogeny of Triatomini (Hemiptera: Reduviidae: Triatominae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tuff Crater Insects
«L» «NZAC_CODE1», «LOC_WIDE», «LOCALITY», «LOC_NARROW», «LOC_Specific» Herbivores found at locality, all observations listed by species within major group 192 Acalitus australis (Lamb, 1952) (Arachnida, Acari: Prostigmata, Eriophyoidea, Eriophyidae) (Puriri erineum mite). Biostatus: endemic CFA1303_N02: record 31/03/2013 leaf erineum seen 208 Aceria calystegiae (Lamb, 1952) (Arachnida, Acari: Prostigmata, Eriophyoidea, Eriophyidae) (Bindweed gall mite). Biostatus: endemic CFA1303_N06: record 31/03/2013 pocket galls common 222 Aceria melicyti Lamb, 1953 (Arachnida, Acari: Prostigmata, Eriophyoidea, Eriophyidae) (Mahoe leaf roll mite). Biostatus: endemic CFA1303_N30: record 31/03/2013 a few leaf edge roll galls seen 241 Eriophyes lambi Manson, 1965 (Arachnida, Acari: Prostigmata, Eriophyoidea, Eriophyidae) (Pohuehue pocket gall mite). Biostatus: endemic CFA1303_N20: record 31/03/2013 pocket galls on leaves 2997 Illeis galbula Mulsant, 1850 (Insecta, Coleoptera, Cucujoidea, Coccinellidae) (Fungus eating ladybird). Biostatus: adventive CFA1303_N04: record 31/03/2013 large larva on puriri leaf, no obvious fungal food 304 Neomycta rubida Broun, 1880 (Insecta, Coleoptera, Curculionoidea, Curculionidae) (Pohutukawa leafminer). Biostatus: endemic CFA1303_N32: record 31/03/2013 holes in new leaves 7 Liriomyza chenopodii (Watt, 1924) (Insecta, Diptera, Opomyzoidea, Agromyzidae) (Australian beet miner). Biostatus: adventive CFA1303_N18: record 31/03/2013 a few narrow leaf mines 9 Liriomyza flavocentralis (Watt, 1923) (Insecta, Diptera, Opomyzoidea, Agromyzidae) (Variable Hebe leafminer). Biostatus: endemic CFA1303_N08: record 31/03/2013 a few mines on shrubs planted near Wharhouse entrance 21 Liriomyza watti Spencer, 1976 (Insecta, Diptera, Opomyzoidea, Agromyzidae) (New Zealand cress leafminer). Biostatus: endemic CFA1303_N07: record 31/03/2013 plant in shade with leaf mines, one leaf with larval parasitoids, larva appears to be white 362 Myrsine shoot tip gall sp. -

Planthopper and Leafhopper Fauna (Hemiptera: Fulgoromorpha Et Cicadomorpha) at Selected Post-Mining Dumping Grounds in Southern Poland
Title: Planthopper and leafhopper fauna (Hemiptera: Fulgoromorpha et Cicadomorpha) at selected post-mining dumping grounds in Southern Poland Author: Marcin Walczak, Mariola Chruściel, Joanna Trela, Klaudia Sojka, Aleksander Herczek Citation style: Walczak Marcin, Chruściel Mariola, Trela Joanna, Sojka Klaudia, Herczek Aleksander. (2019). Planthopper and leafhopper fauna (Hemiptera: Fulgoromorpha et Cicadomorpha) at selected post-mining dumping grounds in Southern Poland. “Annals of the Upper Silesian Museum in Bytom, Entomology” Vol. 28 (2019), s. 1-28, doi 10.5281/zenodo.3564181 ANNALS OF THE UPPER SILESIAN MUSEUM IN BYTOM ENTOMOLOGY Vol. 28 (online 006): 1–28 ISSN 0867-1966, eISSN 2544-039X (online) Bytom, 05.12.2019 MARCIN WALCZAK1 , Mariola ChruśCiel2 , Joanna Trela3 , KLAUDIA SOJKA4 , aleksander herCzek5 Planthopper and leafhopper fauna (Hemiptera: Fulgoromorpha et Cicadomorpha) at selected post- mining dumping grounds in Southern Poland http://doi.org/10.5281/zenodo.3564181 Faculty of Natural Sciences, University of Silesia, Bankowa Str. 9, 40-007 Katowice, Poland 1 e-mail: [email protected]; 2 [email protected]; 3 [email protected] (corresponding author); 4 [email protected]; 5 [email protected] Abstract: The paper presents the results of the study on species diversity and characteristics of planthopper and leafhopper fauna (Hemiptera: Fulgoromorpha et Cicadomorpha) inhabiting selected post-mining dumping grounds in Mysłowice in Southern Poland. The research was conducted in 2014 on several sites located on waste heaps with various levels of insolation and humidity. During the study 79 species were collected. The paper presents the results of ecological analyses complemented by a qualitative analysis performed based on the indices of species diversity. -

Vectors of Chagas Disease, and Implications for Human Health1
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Denisia Jahr/Year: 2006 Band/Volume: 0019 Autor(en)/Author(s): Jurberg Jose, Galvao Cleber Artikel/Article: Biology, ecology, and systematics of Triatominae (Heteroptera, Reduviidae), vectors of Chagas disease, and implications for human health 1095-1116 © Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Biology, ecology, and systematics of Triatominae (Heteroptera, Reduviidae), vectors of Chagas disease, and implications for human health1 J. JURBERG & C. GALVÃO Abstract: The members of the subfamily Triatominae (Heteroptera, Reduviidae) are vectors of Try- panosoma cruzi (CHAGAS 1909), the causative agent of Chagas disease or American trypanosomiasis. As important vectors, triatomine bugs have attracted ongoing attention, and, thus, various aspects of their systematics, biology, ecology, biogeography, and evolution have been studied for decades. In the present paper the authors summarize the current knowledge on the biology, ecology, and systematics of these vectors and discuss the implications for human health. Key words: Chagas disease, Hemiptera, Triatominae, Trypanosoma cruzi, vectors. Historical background (DARWIN 1871; LENT & WYGODZINSKY 1979). The first triatomine bug species was de- scribed scientifically by Carl DE GEER American trypanosomiasis or Chagas (1773), (Fig. 1), but according to LENT & disease was discovered in 1909 under curi- WYGODZINSKY (1979), the first report on as- ous circumstances. In 1907, the Brazilian pects and habits dated back to 1590, by physician Carlos Ribeiro Justiniano das Reginaldo de Lizárraga. While travelling to Chagas (1879-1934) was sent by Oswaldo inspect convents in Peru and Chile, this Cruz to Lassance, a small village in the state priest noticed the presence of large of Minas Gerais, Brazil, to conduct an anti- hematophagous insects that attacked at malaria campaign in the region where a rail- night. -

Leafhoppers, Planthoppers and Psyllids (Hemiptera: Cicadomorpha, Fulgoromorpha, Psylloidea)
ISSN 1211-8788 Acta Musei Moraviae, Scientiae biologicae (Brno) 90: 195–207, 2005 Leafhoppers, planthoppers and psyllids (Hemiptera: Cicadomorpha, Fulgoromorpha, Psylloidea) in ruderal habitats: material attracted by light in the suburbs of Brno (Czech Republic) IGOR MALENOVSKÝ & PAVEL LAUTERER Department of Entomology, Moravian Museum, Hviezdoslavova 29a, 627 00 Brno, Czech Republic; e-mail: [email protected], [email protected] MALENOVSKÝ I. & LAUTERER P. 2005: Leafhoppers, planthoppers and psyllids (Hemiptera: Cicadomorpha, Fulgoromorpha, Psylloidea) in ruderal habitats: material attracted by light in the suburbs of Brno (Czech Republic). Acta Musei Moraviae, Scientiae biologicae (Brno) 90: 195–207. – Leafhoppers, planthoppers and psyllids light-trapped into streetlamps were examined at two sites in complex ruderal habitats (fields, annual and perennial ruderal vegetation, scrub with ruderal and alien species) in Slatina, on the periphery of the city of Brno in South Moravia. A total of 1628 specimens and 61 species were found. Among the dominant species were Empoasca vitis, E. decipiens, E. pteridis, Macrosteles laevis, Psammotettix alienus, Javesella pellucida, Laodelphax striatella, Zyginidia pullula, Kybos lindbergi, and Edwardsiana rosae. Noteworthy are records of Kybos calyculus and Oncopsis appendiculata (both new for the Czech Republic), several rare species living in dry ruderal grassland (Recilia horvathi, Macrosteles quadripunctulatus, Balclutha saltuella), and hygrophilous species caught on dispersal flight (Pentastiridius leporinus, Calamotettix taeniatus, Cicadula placida, Limotettix striola, and Paramesus major). Key words. Hemiptera, Auchenorrhyncha, Psylloidea, ruderal habitats, city fauna, light traps, streetlamps, Czech Republic, South Moravia, faunistics, new records. Introduction Leafhoppers (Cicadomorpha), planthoppers (Fulgoromorpha) and psyllids (Sternorrhyncha: Psylloidea) are phytophagous insects belonging to the order Hemiptera. They suck plant sap, mostly from phloem vessels, some groups feed on xylem or mesophyll tissues. -

Homoptera: Cicadelloidea and Membracoidea) J
Great Basin Naturalist Memoirs Volume 12 Research in the Auchenorrhyncha, Article 6 Homoptera: A Tribute to Paul W. Oman 10-1-1988 Some aspects of the biology, morphology, and evolution of leafhoppers (Homoptera: Cicadelloidea and Membracoidea) J. W. Evans Australian Museum, Sydney, N. S. W., Australia Follow this and additional works at: https://scholarsarchive.byu.edu/gbnm Recommended Citation Evans, J. W. (1988) "Some aspects of the biology, morphology, and evolution of leafhoppers (Homoptera: Cicadelloidea and Membracoidea)," Great Basin Naturalist Memoirs: Vol. 12 , Article 6. Available at: https://scholarsarchive.byu.edu/gbnm/vol12/iss1/6 This Article is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Great Basin Naturalist Memoirs by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. SOME ASPECTS OF THE BIOLOGY, MORPHOLOGY, AND EVOLUTION OF LEAFHOPPERS (HOMOPTERA: CICADELLOIDEA AND MEMBRACOIDEA) J. W. Evans' Abstract —This article summarizes some observations of a varied nature on the biology, morphology, and evolution of the Cicadelloidea (Cicadellidae, Hylicidae, Eurymelidae) and Membracoidea(Membracidae, Aetalionidae, Biturri- tidae, Nicomiidae). These observations, made over a period of more than half a century, have previously been recorded at different times, but lie buried in the literature. It is hoped that their interest will justify repetition and draw attention to some promising lines of research. Biology ulatum Linnaeus (Evans 1946b). In his discus- sion of the function of the songs of various Food Plant Associations Auchenorrhyncha, Ossiannilsson described As Southwood (1961) has pointed out, in- some as being "calls of courtship." Subse- sects have a particularly close association with quently, I noted the presence of well-devel- plants belonging to the predominant flora of oped tymbals in nymphs belonging to every the time. -
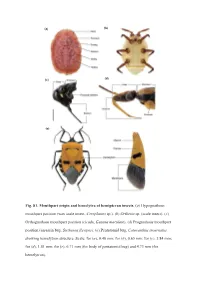
Page 1 (A) (E) Fig. S1. Mouthpart Origin and Hemelytra of Hemipteran
(a) (b) (d) (c) (e) Fig 61. Mouthpart origin and hemelytra of hemipteran insects. (a) Hypognathous mouthpart position (wax scale insect, Ceroplastes sp.). (b) Orthezia sp. (scale insect). (c) Orthognathous mouthpart position (cicada, Gaeana maculate). (d) Prognathous mouthpart position (assassin bug, Sirthenea flavipes). (e) Pentatomid bug, Catacanthus incarnatus showing hemelytron structure. Scale: for (a), 0.40 mm; for (b), 0.65 mm; for (c), 3.84 mm; for (d), 1.81 mm; for (e), 6.71 mm (for body of pentatomid bug) and 4.73 mm (for hemelytron). Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG1 PCG2 Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG3 RNA )LJ62. AliGROOVE analysis for codon positions of protein-coding genes (PCGs) and RNA genes. PCG1, the first codon position of PCGs. PCG2, the second codon position of PCGs. PCG3, the third codon position of PCGs. RNA, sequences of tRNA and rRNA genes. The mean similarity score between sequences is represented by a colored square, based on AliGROOVE scores from -1, indicating great difference in rates from the remainder of the data set, that is, heterogeneity (red coloring), to +1, indicating that ratesmatch all other comparisons (blue coloring). Bactericera sinica Sternorrhyncha Cicadomorpha Coleorrhyncha Fulgoromorpha Dipsocoromorpha Gerromorpha Enicocephalomorpha Nepomorpha Leptopodomorpha Cimicomorpha Heteroptera Pentatomomorpha Eusthenes cupreus FigS33K\ORJHQHWLFWUHHLQIHUUHGIURP3K\OR%D\HVDQDO\VLVRIWKH3&*51$GDWDVHWXQGHUWKe &$7*75PL[WXUHPRGHO9DOXHVDWQRGHVDUH%D\HVLDQ33V -

Bryophyte Ecology Table of Contents
Glime, J. M. 2020. Table of Contents. Bryophyte Ecology. Ebook sponsored by Michigan Technological University 1 and the International Association of Bryologists. Last updated 15 July 2020 and available at <https://digitalcommons.mtu.edu/bryophyte-ecology/>. This file will contain all the volumes, chapters, and headings within chapters to help you find what you want in the book. Once you enter a chapter, there will be a table of contents with clickable page numbers. To search the list, check the upper screen of your pdf reader for a search window or magnifying glass. If there is none, try Ctrl G to open one. TABLE OF CONTENTS BRYOPHYTE ECOLOGY VOLUME 1: PHYSIOLOGICAL ECOLOGY Chapter in Volume 1 1 INTRODUCTION Thinking on a New Scale Adaptations to Land Minimum Size Do Bryophytes Lack Diversity? The "Moss" What's in a Name? Phyla/Divisions Role of Bryology 2 LIFE CYCLES AND MORPHOLOGY 2-1: Meet the Bryophytes Definition of Bryophyte Nomenclature What Makes Bryophytes Unique Who are the Relatives? Two Branches Limitations of Scale Limited by Scale – and No Lignin Limited by Scale – Forced to Be Simple Limited by Scale – Needing to Swim Limited by Scale – and Housing an Embryo Higher Classifications and New Meanings New Meanings for the Term Bryophyte Differences within Bryobiotina 2-2: Life Cycles: Surviving Change The General Bryobiotina Life Cycle Dominant Generation The Life Cycle Life Cycle Controls Generation Time Importance Longevity and Totipotency 2-3: Marchantiophyta Distinguishing Marchantiophyta Elaters Leafy or Thallose? Class -

An Appraisal of the Higher Classification of Cicadas (Hemiptera: Cicadoidea) with Special Reference to the Australian Fauna
© Copyright Australian Museum, 2005 Records of the Australian Museum (2005) Vol. 57: 375–446. ISSN 0067-1975 An Appraisal of the Higher Classification of Cicadas (Hemiptera: Cicadoidea) with Special Reference to the Australian Fauna M.S. MOULDS Australian Museum, 6 College Street, Sydney NSW 2010, Australia [email protected] ABSTRACT. The history of cicada family classification is reviewed and the current status of all previously proposed families and subfamilies summarized. All tribal rankings associated with the Australian fauna are similarly documented. A cladistic analysis of generic relationships has been used to test the validity of currently held views on family and subfamily groupings. The analysis has been based upon an exhaustive study of nymphal and adult morphology, including both external and internal adult structures, and the first comparative study of male and female internal reproductive systems is included. Only two families are justified, the Tettigarctidae and Cicadidae. The latter are here considered to comprise three subfamilies, the Cicadinae, Cicadettinae n.stat. (= Tibicininae auct.) and the Tettigadinae (encompassing the Tibicinini, Platypediidae and Tettigadidae). Of particular note is the transfer of Tibicina Amyot, the type genus of the subfamily Tibicininae, to the subfamily Tettigadinae. The subfamily Plautillinae (containing only the genus Plautilla) is now placed at tribal rank within the Cicadinae. The subtribe Ydiellaria is raised to tribal rank. The American genus Magicicada Davis, previously of the tribe Tibicinini, now falls within the Taphurini. Three new tribes are recognized within the Australian fauna, the Tamasini n.tribe to accommodate Tamasa Distant and Parnkalla Distant, Jassopsaltriini n.tribe to accommodate Jassopsaltria Ashton and Burbungini n.tribe to accommodate Burbunga Distant. -

Homologies of the Head of Membracoidea Based on Nymphal Morphology with Notes on Other Groups of Auchenorrhyncha (Hemiptera)
Eur. J. Entomol. 107: 597–613, 2010 http://www.eje.cz/scripts/viewabstract.php?abstract=1571 ISSN 1210-5759 (print), 1802-8829 (online) Homologies of the head of Membracoidea based on nymphal morphology with notes on other groups of Auchenorrhyncha (Hemiptera) DMITRY A. DMITRIEV Illinois Natural History Survey, Institute of Natural Resource Sustainability at the University of Illinois at Urbana-Champaign, Champaign, Illinois, USA; e-mail: [email protected] Key words. Hemiptera, Membracoidea, Cicadellidae, Cicadoidea, Cercopoidea, Fulgoroidea, head, morphology, ground plan Abstract. The ground plan and comparative morphology of the nymphal head of Membracoidea are presented with particular emphasis on the position of the clypeus, frons, epistomal suture, and ecdysial line. Differences in interpretation of the head structures in Auchenorrhyncha are discussed. Membracoidea head may vary more extensively than heads in any other group of insects. It is often modified by the development of an anterior carina, which apparently was gained and lost multiple times within Membracoidea. The main modifications of the head of Membracoidea and comparison of those changes with the head of other superfamilies of Auchenorrhyncha are described. INTRODUCTION MATERIAL AND METHODS The general morphology of the insect head is relatively Dried and pinned specimens were studied under an Olympus well studied (Ferris, 1942, 1943, 1944; Cook, 1944; SZX12 microscope with SZX-DA drawing tube attachment. DuPorte, 1946; Snodgrass, 1947; Matsuda, 1965; Detailed study of internal structures and boundaries of sclerites Kukalová-Peck, 1985, 1987, 1991, 1992, 2008). There is based on examination of exuviae and specimens cleared in are also a few papers in which the hemipteran head is 5% KOH. -

Arthropods of Public Health Significance in California
ARTHROPODS OF PUBLIC HEALTH SIGNIFICANCE IN CALIFORNIA California Department of Public Health Vector Control Technician Certification Training Manual Category C ARTHROPODS OF PUBLIC HEALTH SIGNIFICANCE IN CALIFORNIA Category C: Arthropods A Training Manual for Vector Control Technician’s Certification Examination Administered by the California Department of Health Services Edited by Richard P. Meyer, Ph.D. and Minoo B. Madon M V C A s s o c i a t i o n of C a l i f o r n i a MOSQUITO and VECTOR CONTROL ASSOCIATION of CALIFORNIA 660 J Street, Suite 480, Sacramento, CA 95814 Date of Publication - 2002 This is a publication of the MOSQUITO and VECTOR CONTROL ASSOCIATION of CALIFORNIA For other MVCAC publications or further informaiton, contact: MVCAC 660 J Street, Suite 480 Sacramento, CA 95814 Telephone: (916) 440-0826 Fax: (916) 442-4182 E-Mail: [email protected] Web Site: http://www.mvcac.org Copyright © MVCAC 2002. All rights reserved. ii Arthropods of Public Health Significance CONTENTS PREFACE ........................................................................................................................................ v DIRECTORY OF CONTRIBUTORS.............................................................................................. vii 1 EPIDEMIOLOGY OF VECTOR-BORNE DISEASES ..................................... Bruce F. Eldridge 1 2 FUNDAMENTALS OF ENTOMOLOGY.......................................................... Richard P. Meyer 11 3 COCKROACHES ........................................................................................... -

Dellapé Et Al.: Cicadomorpha in Citrus Orchards in Argentina 1125
Dellapé et al.: Cicadomorpha in Citrus Orchards in Argentina 1125 DIVERSITY OF CICADOMORPHA (HEMIPTERA: AUCHENORRHYNCHA) IN CITRUS ORCHARDS IN NORTHEASTERN ARGENTINA 1* 2 1 GIMENA DELLAPÉ , JUAN P. BOUVET AND SUSANA L. PARADELL 1División Entomología. Facultad de Ciencias Naturales y Museo, UNLP, Paseo del Bosque s/nº (B1900FWA), La Plata, Buenos Aires, Argentina 2Sección Entomología, INTA EEA Concordia, CC 34 (E3200AQK), Entre Ríos, Argentina *Corresponding autor; E-mail: [email protected] ABSTRACT Among phytophagous insects, the Cicadomorpha are important economically because they damage crops by sucking plant sap and by transmitting plant pathogens, such as Spiro- plasma citri and Xylella fastidiosa to citrus. In Argentina little knowledge exists about this subject. The aim of this work was to study the diversity of Cicadomorpha associated with citrus orchards in Entre Ríos province, and their seasonal fluctuation in relation with cli- matic and phenological conditions. A total of 1,554 specimens belonging to 28 species of Cicadomorpha were collected with yellow sticky traps in sweet orange (Citrus × sinensis (L.) Osbeck) and tangerine (Citrus unshiu Marc) orchards. The Shannon index and the Simpson index suggested a similar trend in the distribution of the dominant species in both crops. In the orange orchard, Cicadomorpha populations increased in the summer coincidently with temperature increases. On the other hand, a significant increase in abundance during the winter months was coincident with increase of early sprouts of the citrus plants. Entre Ríos province represents a new distribution record for 13 species. Tangerine is a newly recorded host-plant for 16 species studied, and eight species are reported for the first time on ‘Valen- cia Late’ orange. -

Example Insect Natural History Data
Example Insect Natural History Data These data were assembled by participants of a workshop held at the University of Florida from May 30 to June 1 of 2018. The data cover all five major insect orders (Coleoptera, Diptera, Hemiptera, Hymenoptera, Lepidoptera) and represent most of the various kinds of natural history information found on insect specimen labels. The data also include representative natural history information from literature sources and online databases. For more information about how these data were assembled and why, see Stucky et al. (2019) __________. Except for works in the public domain, data use licenses are as specified by the original data owners. Coleoptera Example 1 Taxonomy: Coleoptera: Buprestidae: Acmaeodera sp. Record type: database Life stage(s): adult Source: iNaturalist Record URL: https://www.inaturalist.org/observations/12840335 Comments and relevant content: "Feeding on wildflowers in an open meadow in the midlands of South Carolina." Example 2 Taxonomy: Coleoptera: Cerambycidae Record type: literature Source: Paro et al. (2011) Relevant text: "Table 1. Association between girdled and available host-plants (listed alphabetically) and Onciderini beetles in Serra do Japi from 2002 to 2006." The table gives the percentages of each plant species that were girdled along with associated beetle species. Example 3 Taxonomy: Coleoptera: Cerambycidae: Rhaesus serricollis Record type: literature Source: Sama et al. (2010) Relevant text: "Host plants: Polyphagous on deciduous trees like Platanus (Platanaceae), Ficus