Leafhoppers, Planthoppers and Psyllids (Hemiptera: Cicadomorpha, Fulgoromorpha, Psylloidea)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inter-Plant Vibrational Communication in a Leafhopper Insect
Inter-Plant Vibrational Communication in a Leafhopper Insect Anna Eriksson1,2, Gianfranco Anfora1*, Andrea Lucchi2, Meta Virant-Doberlet3, Valerio Mazzoni1 1 Research and Innovation Centre, Fondazione Edmund Mach, San Michele all’Adige, Italy, 2 Department of Coltivazione e Difesa delle Specie Legnose, University of Pisa, Pisa, Italy, 3 Department of Entomology, National Institute of Biology, Ljubljana, Slovenia Abstract Vibrational communication is one of the least understood channels of communication. Most studies have focused on the role of substrate-borne signals in insect mating behavior, where a male and a female establish a stereotyped duet that enables partner recognition and localization. While the effective communication range of substrate-borne signals may be up to several meters, it is generally accepted that insect vibrational communication is limited to a continuous substrate. Until now, interplant communication in absence of physical contact between plants has never been demonstrated in a vibrational communicating insect. With a laser vibrometer we investigated transmission of natural and played back vibrational signals of a grapevine leafhopper, Scaphoideus titanus, when being transmitted between leaves of different cuttings without physical contact. Partners established a vibrational duet up to 6 cm gap width between leaves. Ablation of the antennae showed that antennal mechanoreceptors are not essential in detection of mating signals. Our results demonstrate for the first time that substrate discontinuity does not impose a limitation on communication range of vibrational signals. We also suggest that the behavioral response may depend on the signal intensity. Citation: Eriksson A, Anfora G, Lucchi A, Virant-Doberlet M, Mazzoni V (2011) Inter-Plant Vibrational Communication in a Leafhopper Insect. -

The Influence of Prairie Restoration on Hemiptera
CAN THE ONE TRUE BUG BE THE ONE TRUE ANSWER? THE INFLUENCE OF PRAIRIE RESTORATION ON HEMIPTERA COMPOSITION Thesis Submitted to The College of Arts and Sciences of the UNIVERSITY OF DAYTON In Partial Fulfillment of the Requirements for The Degree of Master of Science in Biology By Stephanie Kay Gunter, B.A. Dayton, Ohio August 2021 CAN THE ONE TRUE BUG BE THE ONE TRUE ANSWER? THE INFLUENCE OF PRAIRIE RESTORATION ON HEMIPTERA COMPOSITION Name: Gunter, Stephanie Kay APPROVED BY: Chelse M. Prather, Ph.D. Faculty Advisor Associate Professor Department of Biology Ryan W. McEwan, Ph.D. Committee Member Associate Professor Department of Biology Mark G. Nielsen Ph.D. Committee Member Associate Professor Department of Biology ii © Copyright by Stephanie Kay Gunter All rights reserved 2021 iii ABSTRACT CAN THE ONE TRUE BUG BE THE ONE TRUE ANSWER? THE INFLUENCE OF PRAIRIE RESTORATION ON HEMIPTERA COMPOSITION Name: Gunter, Stephanie Kay University of Dayton Advisor: Dr. Chelse M. Prather Ohio historically hosted a patchwork of tallgrass prairies, which provided habitat for native species and prevented erosion. As these vulnerable habitats have declined in the last 200 years due to increased human land use, restorations of these ecosystems have increased, and it is important to evaluate their success. The Hemiptera (true bugs) are an abundant and varied order of insects including leafhoppers, aphids, cicadas, stink bugs, and more. They play important roles in grassland ecosystems, feeding on plant sap and providing prey to predators. Hemipteran abundance and composition can respond to grassland restorations, age of restoration, and size and isolation of habitat. -

Planthopper and Leafhopper Fauna (Hemiptera: Fulgoromorpha Et Cicadomorpha) at Selected Post-Mining Dumping Grounds in Southern Poland
Title: Planthopper and leafhopper fauna (Hemiptera: Fulgoromorpha et Cicadomorpha) at selected post-mining dumping grounds in Southern Poland Author: Marcin Walczak, Mariola Chruściel, Joanna Trela, Klaudia Sojka, Aleksander Herczek Citation style: Walczak Marcin, Chruściel Mariola, Trela Joanna, Sojka Klaudia, Herczek Aleksander. (2019). Planthopper and leafhopper fauna (Hemiptera: Fulgoromorpha et Cicadomorpha) at selected post-mining dumping grounds in Southern Poland. “Annals of the Upper Silesian Museum in Bytom, Entomology” Vol. 28 (2019), s. 1-28, doi 10.5281/zenodo.3564181 ANNALS OF THE UPPER SILESIAN MUSEUM IN BYTOM ENTOMOLOGY Vol. 28 (online 006): 1–28 ISSN 0867-1966, eISSN 2544-039X (online) Bytom, 05.12.2019 MARCIN WALCZAK1 , Mariola ChruśCiel2 , Joanna Trela3 , KLAUDIA SOJKA4 , aleksander herCzek5 Planthopper and leafhopper fauna (Hemiptera: Fulgoromorpha et Cicadomorpha) at selected post- mining dumping grounds in Southern Poland http://doi.org/10.5281/zenodo.3564181 Faculty of Natural Sciences, University of Silesia, Bankowa Str. 9, 40-007 Katowice, Poland 1 e-mail: [email protected]; 2 [email protected]; 3 [email protected] (corresponding author); 4 [email protected]; 5 [email protected] Abstract: The paper presents the results of the study on species diversity and characteristics of planthopper and leafhopper fauna (Hemiptera: Fulgoromorpha et Cicadomorpha) inhabiting selected post-mining dumping grounds in Mysłowice in Southern Poland. The research was conducted in 2014 on several sites located on waste heaps with various levels of insolation and humidity. During the study 79 species were collected. The paper presents the results of ecological analyses complemented by a qualitative analysis performed based on the indices of species diversity. -

Preliminary List of Auchenorrhyncha with Notes on Distribution and Impnrtanee of Saecles in .Iurkey, XIII
Türk. mt. Kor. Derg. (1984) 8: 33-44 Preliminary list of Auchenorrhyncha with notes on distribution and impnrtanee of saecles in .Iurkey, XIII. Family Gicadellidae Typhlocybinae : Typhlocybini ( Part i ) Nlyazd LODOS* Ayla KALKANDELEN** Summary Turkish fauna of Typhloeybini, excluding Eupteryx species, is represented by 19 species of 9 genera as a re su lt of this study. Seven newly recorded species are: Edwardsiana avellanae (Edw.), E. flavescens (F.), E. sallcioola (Edw.), Eupteryeyba jueunda (H.S.), Ficoeyba fiearia (Horv.) , Typhloeyba quereus (F.) and Aguriahana germart (Zett.). Distribution, abundanee and the plants on which the specimens were eolleeted of eaeh species are given. Introduction Turkish T!yphlocilbini was not studied as a whole up to date. Howe ver, only 12 species of this group have been reported previouslv by se veral workers, The Hrst record made by Fahringer (1922) was Ed wardsiana Iethierryi (Edw.). Metcalf (1968) also Iisted this species from Turkeyaccording to Fahringer (1.c.). Dlabola (1957) reported Ribautiana ognevi (Zach.) trom Turkey tınder the name of 'Iyphlocyba horvathiana Dlab, Linnavuori (1965) also recorded such species as Linnavuoriana sexmaculata (Hardy), Youngiada tarsalis Linn. and Zygineı1la pulchra (Löw) from Turkey. Ural et al. (1973) recorded Edwardsiana spinigera (Edw.) during the taunistic study in hazel orchards in eastern Black Sea Coast. Dlabola (1971, 1981) reported Edwardsiana rosae (L,.) , E. tshlrıari Zach., Ribautiana alces (Rıb.), R. tenerrima (RS.) and Younglada pandellei (Leth.) addrtionally in his publicatdons, * University of Ege, Faculty of Agriculture, Plant Protection Department, Bornova, İzmir-Turkey. ** Plant Proteetion Researeh Institute, Plant Protection Museum, Kalaba, Ankara Turkey. Almış (Received) : 16.9.1982 33 In this group of typhlocybids, especially E. -

A New Family of Coreoidea from the Lower Cretaceous Lebanese Amber (Hemiptera: Pentatomomorpha)
P O L I S H JOUR NAL OF ENTOMOLO G Y POLSKIE PISMO ENTOMOL OGICZ N E VOL. 80: 627-644 Gdynia 31 December 2011 DOI: 10.2478/v10200-011-0049-5 A new family of Coreoidea from the Lower Cretaceous Lebanese Amber (Hemiptera: Pentatomomorpha) DANY AZAR1, ANDRÉ NEL2, MICHAEL S. ENGEL3, 4, ROMAIN GARROUSTE2, ARMAND MATOCQ2 1Lebanese University, Faculty of Sciences II, Department of Natural Sciences, PO box 26110217, Fanar – Matn, Lebanon, e-mail: [email protected]; 2CNRS UMR 7205, CP 50, Entomologie, Muséum National d'Histoire Naturelle, 45 rue Buffon, F-75005 Paris, France, e-mails: [email protected], [email protected], [email protected]; 3Division of Entomology (Paleoentomology), Natural History Museum, University of Kansas, Lawrence, KS 66049-2811, USA, e-mail: [email protected]; 4Department of Ecology & Evolutionary Biology, 1501 Crestline Drive – Suite 140, University of Kansas, Lawrence, KS 66049-2811, USA ABSTRACT. A new genus and species, Yuripopovina magnifica, belonging to a new coreoid family, Yuripopovinidae (Hemiptera: Pentatomomorpha), is described and illustrated from the Lower Cretaceous amber of Lebanon. The species represents the first definitive Mesozoic record for the Coreoidea. A cladistic analysis of Coreoidea, including the new family, is undertaken. KEY WORDS: Pentatomomorpha, Coreoidea, Yuripopovinidae, fam. n., gen. n., sp. n., Lebanon, phylogeny. INTRODUCTION The Pentatomomorpha with its 14 000 known living species (WEIRAUCH & SCHUH 2011) is the second largest of the seven heteropteran infraorders (SCHAEFER 1993, ŠTYS & KERZHNER 1975) (Enicocephalomorpha, Dipsocoromorpha, Gerromorpha, Nepomorpha, Leptodomorpha, Cimicomorpha, and Pentatomorpha). Most authors recognize five superfamilies within Pentatomomorpha, but there remains controversy regarding the 628 Polish Journal of Entomology 80 (4) composition of these superfamilies (SCHAEFER 1993, ŠTYS 1961). -

Diversity and Abundance of Insect Herbivores Foraging on Seedlings in a Rainforest in Guyana
R Ecological Entomology (1999) 24, 245±259 Diversity and abundance of insect herbivores foraging on seedlings in a rainforest in Guyana YVES BASSET CABI Bioscience: Environment, Ascot, U.K. Abstract. 1. Free-living insect herbivores foraging on 10 000 tagged seedlings representing ®ve species of common rainforest trees were surveyed monthly for more than 1 year in an unlogged forest plot of 1 km2 in Guyana. 2. Overall, 9056 insect specimens were collected. Most were sap-sucking insects, which represented at least 244 species belonging to 25 families. Leaf-chewing insects included at least 101 species belonging to 16 families. Herbivore densities were among the lowest densities reported in tropical rainforests to date: 2.4 individuals per square metre of foliage. 3. Insect host speci®city was assessed by calculating Lloyd's index of patchiness from distributional records and considering feeding records in captivity and in situ. Generalists represented 84 and 78% of sap-sucking species and individuals, and 75 and 42% of leaf-chewing species and individuals. In particular, several species of polyphagous xylem-feeding Cicadellinae were strikingly abundant on all hosts. 4. The high incidence of generalist insects suggests that the Janzen±Connell model, explaining rates of attack on seedlings as a density-dependent process resulting from contagion of specialist insects from parent trees, is unlikely to be valid in this study system. 5. Given the rarity of ¯ushing events for the seedlings during the study period, the low insect densities, and the high proportion of generalists, the data also suggest that seedlings may represent a poor resource for free-living insect herbivores in rainforests. -

A Checklist of the Auchenorrhyncha of Belarus (Hemiptera, Fulgoromorpha Et Cicadomorpha)
Beiträge zur Zikadenkunde 7: 29-47 (2004) 29 A checklist of the Auchenorrhyncha of Belarus (Hemiptera, Fulgoromorpha et Cicadomorpha) Oleg Borodin1 Kurzfassung: Es wird eine Artenliste der Zikaden von Weißrußland präsentiert, mit An- gaben zu Vorkommen in einzelnen Regionen, Habitat- und Feuchtepräferenzen, Lebens- formen der Nährpflanzen und Phänologie. Die Liste enthält 331 Arten aus 10 Familien. Abstract: A checklist of the Auchenorrhyncha species of Belarus is provided, with data of their occurrence in geographic provinces, habitat and moisture preferences, life forms of food plants, and phenology. The list includes 331 species belonging to 10 families. Key words: Auchenorrhyncha, checklist, Belarus, phytophagous insects 1. Introduction For a long time the Auchenorrhyncha have been one of the least studied insect groups of Belarus. Nast (1972, 1987) lists only 7 species for the whole country, although early studies were carried out in 1925-1926 (Yazentkovskij 1925; Bryanzev 1926; Solowiew 1926). These works, including a number of successive studies carried out through the Belorussian Agricul- tural Academy (Dubrovskaya & Kovaleva 1970; Kovaleva 1970) and the Institute of Plant Protection have applied character and include only a few species which are important for agriculture. Other studies contain information about Auchenorrhyncha communities (Yaki- movich 1982; Chumakov 1986). Furthermore, some species were recorded by foreign col- leagues, e.g. Cicadella lasiocarpae Oss. (Dmitriev 1998), Coryphaelus gyllenhalii (Fall.) and Anake- lisia fasciata (Kbm.) (Nast 1976). The total number of published species in all these papers is only 71. The present paper provides an up-to-date checklist based on new studies and collec- tions carried out by the author since 1998, mainly in western and central parts of the country. -

Kenai National Wildlife Refuge Species List, Version 2018-07-24
Kenai National Wildlife Refuge Species List, version 2018-07-24 Kenai National Wildlife Refuge biology staff July 24, 2018 2 Cover image: map of 16,213 georeferenced occurrence records included in the checklist. Contents Contents 3 Introduction 5 Purpose............................................................ 5 About the list......................................................... 5 Acknowledgments....................................................... 5 Native species 7 Vertebrates .......................................................... 7 Invertebrates ......................................................... 55 Vascular Plants........................................................ 91 Bryophytes ..........................................................164 Other Plants .........................................................171 Chromista...........................................................171 Fungi .............................................................173 Protozoans ..........................................................186 Non-native species 187 Vertebrates ..........................................................187 Invertebrates .........................................................187 Vascular Plants........................................................190 Extirpated species 207 Vertebrates ..........................................................207 Vascular Plants........................................................207 Change log 211 References 213 Index 215 3 Introduction Purpose to avoid implying -

Planthopper and Leafhopper Fauna (Hemiptera: Fulgoromorpha Et
ANNALS OF THE UPPER SILESIAN MUSEUM IN BYTOM ENTOMOLOGY Vol. 28 (online 006): 1–28 ISSN 0867-1966, eISSN 2544-039X (online) Bytom, 05.12.2019 MARCIN WALCZAK1 , Mariola ChruśCiel2 , Joanna Trela3 , KLAUDIA SOJKA4 , aleksander herCzek5 Planthopper and leafhopper fauna (Hemiptera: Fulgoromorpha et Cicadomorpha) at selected post- mining dumping grounds in Southern Poland http://doi.org/10.5281/zenodo.3564181 Faculty of Natural Sciences, University of Silesia, Bankowa Str. 9, 40-007 Katowice, Poland 1 e-mail: [email protected]; 2 [email protected]; 3 [email protected] (corresponding author); 4 [email protected]; 5 [email protected] Abstract: The paper presents the results of the study on species diversity and characteristics of planthopper and leafhopper fauna (Hemiptera: Fulgoromorpha et Cicadomorpha) inhabiting selected post-mining dumping grounds in Mysłowice in Southern Poland. The research was conducted in 2014 on several sites located on waste heaps with various levels of insolation and humidity. During the study 79 species were collected. The paper presents the results of ecological analyses complemented by a qualitative analysis performed based on the indices of species diversity. Key words: insects communities, zoocenological analyses, dominant species, seasonal dynamics of abundance, ecology, distribution, synanthropy, post-industrial areas, biodiversity in degraded environments, anthropopressure, natural succession. INTRODUCTION Planthoppers and leafhoppers (Hemiptera: Fulgoromorpha et Cicadomorpha) are phytophagous insects which are highly related to their host plants, and most of them are trophically specialized as mono- or oligophagous (niCkel 2003), so most of them are attached to the specific plant associations, where they form multispecies communities. -
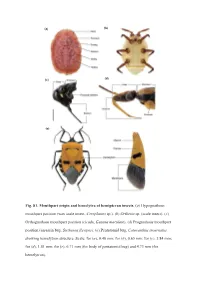
Page 1 (A) (E) Fig. S1. Mouthpart Origin and Hemelytra of Hemipteran
(a) (b) (d) (c) (e) Fig 61. Mouthpart origin and hemelytra of hemipteran insects. (a) Hypognathous mouthpart position (wax scale insect, Ceroplastes sp.). (b) Orthezia sp. (scale insect). (c) Orthognathous mouthpart position (cicada, Gaeana maculate). (d) Prognathous mouthpart position (assassin bug, Sirthenea flavipes). (e) Pentatomid bug, Catacanthus incarnatus showing hemelytron structure. Scale: for (a), 0.40 mm; for (b), 0.65 mm; for (c), 3.84 mm; for (d), 1.81 mm; for (e), 6.71 mm (for body of pentatomid bug) and 4.73 mm (for hemelytron). Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG1 PCG2 Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG3 RNA )LJ62. AliGROOVE analysis for codon positions of protein-coding genes (PCGs) and RNA genes. PCG1, the first codon position of PCGs. PCG2, the second codon position of PCGs. PCG3, the third codon position of PCGs. RNA, sequences of tRNA and rRNA genes. The mean similarity score between sequences is represented by a colored square, based on AliGROOVE scores from -1, indicating great difference in rates from the remainder of the data set, that is, heterogeneity (red coloring), to +1, indicating that ratesmatch all other comparisons (blue coloring). Bactericera sinica Sternorrhyncha Cicadomorpha Coleorrhyncha Fulgoromorpha Dipsocoromorpha Gerromorpha Enicocephalomorpha Nepomorpha Leptopodomorpha Cimicomorpha Heteroptera Pentatomomorpha Eusthenes cupreus FigS33K\ORJHQHWLFWUHHLQIHUUHGIURP3K\OR%D\HVDQDO\VLVRIWKH3&*51$GDWDVHWXQGHUWKe &$7*75PL[WXUHPRGHO9DOXHVDWQRGHVDUH%D\HVLDQ33V -

Insect Pests of Wheat Crop at Tandojam
Journal of Entomology and Zoology Studies 2019; 7(1): 1317-1320 E-ISSN: 2320-7078 P-ISSN: 2349-6800 Insect pests of wheat crop at Tandojam JEZS 2019; 7(1): 1317-1320 © 2019 JEZS Received: 11-11-2018 Accepted: 14-12-2018 Muhammad Umar Brohi, Imran Khatri, Arfan Ahmed Gilal, Zubair Ahmed, Zamin Hussain Dahri and Ihasan Ahmed Magssi Muhammad Umar Brohi Department of Entomology, Sindh Agriculture University Abstract Tandojam, Sindh, Pakistan The present study was conducted to study the insect pest diversity on a wheat crop, the specimens were collected from wheat crop adjacent to the Administration Block Sindh Agriculture University Tandojam Imran Khatri from 15th of January 2018 to 25th of March 2018. In present study total 13 insect pest species were Department of Entomology, discovered under four orders, Hemiptera, Linnaeus 1758 revealed 10 species, Nilaparvata lugens (Stål, Sindh Agriculture University 1854) and Delphacodes kuscheli Fennah, 1955 under family Delphacidae Leach, Pentastiridius hodgarti Tandojam, Sindh, Pakistan (Distant, 1911) under family Cixiidae Spinola, 1839. Collection of leafhoppers revealed the occurrence Arfan Ahmed Gilal of 3 species Balclutha incisa (Matsumura, 1902) under family Cicadellidae, Latreille 1802, Psammotettix Department of Entomology, emarginata, Empoasca punjabensis Singh-Pruthi, 1940. One aphid species Aphis gossypii Glover, 1877 Sindh Agriculture University under family Aphididae Latreille, 1802. True bugs were found with 3 species Scotinophara limosa Tandojam, Sindh, Pakistan (Walker, 1867) family Pentatomidae Leach, 1815, two species under family Lygaeidae Schilling, 1829, Graptostethus servus (Fabricius, 1787) and Oxycarenus hyalinipennis (Costa, 1843). One moth Zubair Ahmed Helicoverpa armigera (Hübner, 1808) under family Noctuidae, Latreille, 1809. One grasshopper species Department of Zoology, Federal Acrida exaltata Walker, 1859 under family Acrididae MacLeay, 1821. -

Bryophyte Ecology Table of Contents
Glime, J. M. 2020. Table of Contents. Bryophyte Ecology. Ebook sponsored by Michigan Technological University 1 and the International Association of Bryologists. Last updated 15 July 2020 and available at <https://digitalcommons.mtu.edu/bryophyte-ecology/>. This file will contain all the volumes, chapters, and headings within chapters to help you find what you want in the book. Once you enter a chapter, there will be a table of contents with clickable page numbers. To search the list, check the upper screen of your pdf reader for a search window or magnifying glass. If there is none, try Ctrl G to open one. TABLE OF CONTENTS BRYOPHYTE ECOLOGY VOLUME 1: PHYSIOLOGICAL ECOLOGY Chapter in Volume 1 1 INTRODUCTION Thinking on a New Scale Adaptations to Land Minimum Size Do Bryophytes Lack Diversity? The "Moss" What's in a Name? Phyla/Divisions Role of Bryology 2 LIFE CYCLES AND MORPHOLOGY 2-1: Meet the Bryophytes Definition of Bryophyte Nomenclature What Makes Bryophytes Unique Who are the Relatives? Two Branches Limitations of Scale Limited by Scale – and No Lignin Limited by Scale – Forced to Be Simple Limited by Scale – Needing to Swim Limited by Scale – and Housing an Embryo Higher Classifications and New Meanings New Meanings for the Term Bryophyte Differences within Bryobiotina 2-2: Life Cycles: Surviving Change The General Bryobiotina Life Cycle Dominant Generation The Life Cycle Life Cycle Controls Generation Time Importance Longevity and Totipotency 2-3: Marchantiophyta Distinguishing Marchantiophyta Elaters Leafy or Thallose? Class