Opening Keynote Talks I-1 Fossils Versus Molecules and Cladistics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Zealand's Genetic Diversity
1.13 NEW ZEALAND’S GENETIC DIVERSITY NEW ZEALAND’S GENETIC DIVERSITY Dennis P. Gordon National Institute of Water and Atmospheric Research, Private Bag 14901, Kilbirnie, Wellington 6022, New Zealand ABSTRACT: The known genetic diversity represented by the New Zealand biota is reviewed and summarised, largely based on a recently published New Zealand inventory of biodiversity. All kingdoms and eukaryote phyla are covered, updated to refl ect the latest phylogenetic view of Eukaryota. The total known biota comprises a nominal 57 406 species (c. 48 640 described). Subtraction of the 4889 naturalised-alien species gives a biota of 52 517 native species. A minimum (the status of a number of the unnamed species is uncertain) of 27 380 (52%) of these species are endemic (cf. 26% for Fungi, 38% for all marine species, 46% for marine Animalia, 68% for all Animalia, 78% for vascular plants and 91% for terrestrial Animalia). In passing, examples are given both of the roles of the major taxa in providing ecosystem services and of the use of genetic resources in the New Zealand economy. Key words: Animalia, Chromista, freshwater, Fungi, genetic diversity, marine, New Zealand, Prokaryota, Protozoa, terrestrial. INTRODUCTION Article 10b of the CBD calls for signatories to ‘Adopt The original brief for this chapter was to review New Zealand’s measures relating to the use of biological resources [i.e. genetic genetic resources. The OECD defi nition of genetic resources resources] to avoid or minimize adverse impacts on biological is ‘genetic material of plants, animals or micro-organisms of diversity [e.g. genetic diversity]’ (my parentheses). -

Planthopper and Leafhopper Fauna (Hemiptera: Fulgoromorpha Et Cicadomorpha) at Selected Post-Mining Dumping Grounds in Southern Poland
Title: Planthopper and leafhopper fauna (Hemiptera: Fulgoromorpha et Cicadomorpha) at selected post-mining dumping grounds in Southern Poland Author: Marcin Walczak, Mariola Chruściel, Joanna Trela, Klaudia Sojka, Aleksander Herczek Citation style: Walczak Marcin, Chruściel Mariola, Trela Joanna, Sojka Klaudia, Herczek Aleksander. (2019). Planthopper and leafhopper fauna (Hemiptera: Fulgoromorpha et Cicadomorpha) at selected post-mining dumping grounds in Southern Poland. “Annals of the Upper Silesian Museum in Bytom, Entomology” Vol. 28 (2019), s. 1-28, doi 10.5281/zenodo.3564181 ANNALS OF THE UPPER SILESIAN MUSEUM IN BYTOM ENTOMOLOGY Vol. 28 (online 006): 1–28 ISSN 0867-1966, eISSN 2544-039X (online) Bytom, 05.12.2019 MARCIN WALCZAK1 , Mariola ChruśCiel2 , Joanna Trela3 , KLAUDIA SOJKA4 , aleksander herCzek5 Planthopper and leafhopper fauna (Hemiptera: Fulgoromorpha et Cicadomorpha) at selected post- mining dumping grounds in Southern Poland http://doi.org/10.5281/zenodo.3564181 Faculty of Natural Sciences, University of Silesia, Bankowa Str. 9, 40-007 Katowice, Poland 1 e-mail: [email protected]; 2 [email protected]; 3 [email protected] (corresponding author); 4 [email protected]; 5 [email protected] Abstract: The paper presents the results of the study on species diversity and characteristics of planthopper and leafhopper fauna (Hemiptera: Fulgoromorpha et Cicadomorpha) inhabiting selected post-mining dumping grounds in Mysłowice in Southern Poland. The research was conducted in 2014 on several sites located on waste heaps with various levels of insolation and humidity. During the study 79 species were collected. The paper presents the results of ecological analyses complemented by a qualitative analysis performed based on the indices of species diversity. -

Higherlevel Phylogeny of the Insect Order Hemiptera
Systematic Entomology (2011), DOI: 10.1111/j.1365-3113.2011.00611.x Higher-level phylogeny of the insect order Hemiptera: is Auchenorrhyncha really paraphyletic? JASON R. CRYAN and JULIE M. URBAN Laboratory for Conservation and Evolutionary Genetics, New York State Museum, Albany, NY, U.S.A. Abstract. The higher-level phylogeny of the order Hemiptera remains a contentious topic in insect systematics. The controversy is chiefly centred on the unresolved question of whether or not the hemipteran suborder Auchenorrhyncha (including the extant superfamilies Fulgoroidea, Membracoidea, Cicadoidea and Cercopoidea) is a monophyletic lineage. Presented here are the results of a multilocus molecular phylogenetic investigation of relationships among the major hemipteran lineages, designed specifically to address the question of Auchenorrhyncha monophyly in the context of broad taxonomic sampling across Hemiptera. Phylogenetic analyses (maximum parsimony, maximum likelihood and Bayesian inference) were based on DNA nucleotide sequence data from seven gene regions (18S rDNA, 28S rDNA, histone H3, histone 2A, wingless, cytochrome c oxidase I and NADH dehydrogenase subunit 4 ) generated from 86 in-group exemplars representing all major lineages of Hemiptera (plus seven out-group taxa). All combined analyses of these data recover the monophyly of Auchenorrhyncha, and also support the monophyly of each of the following lineages: Hemiptera, Sternorrhyncha, Heteropterodea, Heteroptera, Fulgoroidea, Cicadomorpha, Membracoidea, Cercopoidea and Cicadoidea. Also -

Leafhoppers, Planthoppers and Psyllids (Hemiptera: Cicadomorpha, Fulgoromorpha, Psylloidea)
ISSN 1211-8788 Acta Musei Moraviae, Scientiae biologicae (Brno) 90: 195–207, 2005 Leafhoppers, planthoppers and psyllids (Hemiptera: Cicadomorpha, Fulgoromorpha, Psylloidea) in ruderal habitats: material attracted by light in the suburbs of Brno (Czech Republic) IGOR MALENOVSKÝ & PAVEL LAUTERER Department of Entomology, Moravian Museum, Hviezdoslavova 29a, 627 00 Brno, Czech Republic; e-mail: [email protected], [email protected] MALENOVSKÝ I. & LAUTERER P. 2005: Leafhoppers, planthoppers and psyllids (Hemiptera: Cicadomorpha, Fulgoromorpha, Psylloidea) in ruderal habitats: material attracted by light in the suburbs of Brno (Czech Republic). Acta Musei Moraviae, Scientiae biologicae (Brno) 90: 195–207. – Leafhoppers, planthoppers and psyllids light-trapped into streetlamps were examined at two sites in complex ruderal habitats (fields, annual and perennial ruderal vegetation, scrub with ruderal and alien species) in Slatina, on the periphery of the city of Brno in South Moravia. A total of 1628 specimens and 61 species were found. Among the dominant species were Empoasca vitis, E. decipiens, E. pteridis, Macrosteles laevis, Psammotettix alienus, Javesella pellucida, Laodelphax striatella, Zyginidia pullula, Kybos lindbergi, and Edwardsiana rosae. Noteworthy are records of Kybos calyculus and Oncopsis appendiculata (both new for the Czech Republic), several rare species living in dry ruderal grassland (Recilia horvathi, Macrosteles quadripunctulatus, Balclutha saltuella), and hygrophilous species caught on dispersal flight (Pentastiridius leporinus, Calamotettix taeniatus, Cicadula placida, Limotettix striola, and Paramesus major). Key words. Hemiptera, Auchenorrhyncha, Psylloidea, ruderal habitats, city fauna, light traps, streetlamps, Czech Republic, South Moravia, faunistics, new records. Introduction Leafhoppers (Cicadomorpha), planthoppers (Fulgoromorpha) and psyllids (Sternorrhyncha: Psylloidea) are phytophagous insects belonging to the order Hemiptera. They suck plant sap, mostly from phloem vessels, some groups feed on xylem or mesophyll tissues. -

Homoptera: Fulgoroidea: Jubisentidae)
Russian Entomol. J. 29(1): 6–11 © RUSSIAN ENTOMOLOGICAL JOURNAL, 2020 The earliest fully brachypterous auchenorrhynchan from Cretaceous Burmese amber (Homoptera: Fulgoroidea: Jubisentidae) Äðåâíåéøàÿ öèêàäêà ñ ñèëüíî óêîðî÷åííûìè êðûëüÿìè èç ìåëîâîãî áèðìàíñêîãî ÿíòàðÿ (Homoptera: Fulgoroidea: Jubisentidae) Dmitry E. Shcherbakov Ä.Å. Ùåðáàêîâ Borissiak Paleontological Institute, Russian Academy of Sciences, Moscow, Russia; [email protected]. Палеонтологический институт им. А.А. Борисяка РАН, Москва, Россия. KEY WORDS: planthoppers, Perforissidae, wing dimorphism, brachyptery, sensory pits, phylogeny, fossil, host plants, grasses, camouflage, mimicry. КЛЮЧЕВЫЕ СЛОВА: носатки, Perforissidae, крыловой диморфизм, короткокрылость, сенсорные ямки, филогения, ископаемые, кормовые растения, травы, маскировка, мимикрия. ABSTRACT. Psilargus anufrievi gen. et sp.n. (Psi- taceous Lagerstätten. Among many wonderful and un- larginae subfam.n.) from mid-Cretaceous Burmese expected insect taxa, three endemic planthopper fami- amber is assigned to the family Jubisentidae in basal lies have recently been discovered in Burmese amber — (pre-cixioid) Fulgoroidea. The two formerly known Dorytocidae, Yetkhatidae and Jubisentidae [Emeljan- genera of this family are placed in Jubisentinae stat.n. ov, Shcherbakov, 2018; Song et al., 2019; Zhang et al., The only known specimen of the new species is a minute 2019]. In the Burmese amber fauna these groups coexist female with extremely shortened wings. It is the earliest with widespread Cretaceous families, such as Perforis- recorded instance of extreme brachyptery in Auchenor- sidae [Shcherbakov, 2007a; Zhang et al., 2017] and rhyncha. All known Jubisentidae were flightless, cam- Mimarachnidae [Shcherbakov, 2007b, 2017; Luo et al., ouflaged, and likely associated with herbs in the Bur- 2020; etc.], and several extant families, such as Cixiidae mese Cretaceous tropics. -

The Complete Mitochondrial Genome of Four Hylicinae (Hemiptera: Cicadellidae): Structural Features and Phylogenetic Implications
insects Article The Complete Mitochondrial Genome of Four Hylicinae (Hemiptera: Cicadellidae): Structural Features and Phylogenetic Implications Jiu Tang y , Weijian Huang y and Yalin Zhang * Key Laboratory of Plant Protection Resources and Pest Management, Ministry of Education, Entomological Museum, College of Plant Protection, Northwest A&F University, Yangling 712100, China; [email protected] (J.T.); [email protected] (W.H.) * Correspondence: [email protected]; Tel.: +86-029-87092190 These two authors contributed equally in this study. y Received: 19 November 2020; Accepted: 4 December 2020; Published: 7 December 2020 Simple Summary: Hylicinae, containing 43 described species in 13 genera of two tribes, is one of the most morphologically unique subfamilies of Cicadellidae. Phylogenetic studies on this subfamily were mainly based on morphological characters or several gene fragments and just involved single or two taxa. No mitochondrial genome was reported in Hylicinae before. Therefore, we sequenced and analyzed four complete mtgenomes of Hylicinae (Nacolus tuberculatus, Hylica paradoxa, Balala fujiana, and Kalasha nativa) for the first time to reveal mtgenome characterizations and reconstruct phylogenetic relationships of this group. The comparative analyses showed the mtgenome characterizations of Hylicinae are similar to members of Membracoidea. In phylogenetic results, Hylicinae was recovered as a monophyletic group in Cicadellidae and formed to the sister group of Coelidiinae + Iassinae. These results provide the comprehensive framework and worthy information toward the future researches of this subfamily. Abstract: To reveal mtgenome characterizations and reconstruct phylogenetic relationships of Hylicinae, the complete mtgenomes of four hylicine species, including Nacolus tuberculatus, Hylica paradoxa, Balala fujiana, and Kalasha nativa, were sequenced and comparatively analyzed for the first time. -

ARTHROPODA Subphylum Hexapoda Protura, Springtails, Diplura, and Insects
NINE Phylum ARTHROPODA SUBPHYLUM HEXAPODA Protura, springtails, Diplura, and insects ROD P. MACFARLANE, PETER A. MADDISON, IAN G. ANDREW, JOCELYN A. BERRY, PETER M. JOHNS, ROBERT J. B. HOARE, MARIE-CLAUDE LARIVIÈRE, PENELOPE GREENSLADE, ROSA C. HENDERSON, COURTenaY N. SMITHERS, RicarDO L. PALMA, JOHN B. WARD, ROBERT L. C. PILGRIM, DaVID R. TOWNS, IAN McLELLAN, DAVID A. J. TEULON, TERRY R. HITCHINGS, VICTOR F. EASTOP, NICHOLAS A. MARTIN, MURRAY J. FLETCHER, MARLON A. W. STUFKENS, PAMELA J. DALE, Daniel BURCKHARDT, THOMAS R. BUCKLEY, STEVEN A. TREWICK defining feature of the Hexapoda, as the name suggests, is six legs. Also, the body comprises a head, thorax, and abdomen. The number A of abdominal segments varies, however; there are only six in the Collembola (springtails), 9–12 in the Protura, and 10 in the Diplura, whereas in all other hexapods there are strictly 11. Insects are now regarded as comprising only those hexapods with 11 abdominal segments. Whereas crustaceans are the dominant group of arthropods in the sea, hexapods prevail on land, in numbers and biomass. Altogether, the Hexapoda constitutes the most diverse group of animals – the estimated number of described species worldwide is just over 900,000, with the beetles (order Coleoptera) comprising more than a third of these. Today, the Hexapoda is considered to contain four classes – the Insecta, and the Protura, Collembola, and Diplura. The latter three classes were formerly allied with the insect orders Archaeognatha (jumping bristletails) and Thysanura (silverfish) as the insect subclass Apterygota (‘wingless’). The Apterygota is now regarded as an artificial assemblage (Bitsch & Bitsch 2000). -

Off-Target Capture Data, Endosymbiont Genes and Morphology Reveal A
Biological Journal of the Linnean Society, 2019, 128, 865–886. With 7 figures. Off-target capture data, endosymbiont genes and morphology reveal a relict lineage that is sister to all Downloaded from https://academic.oup.com/biolinnean/article-abstract/128/4/865/5586699 by [email protected] on 06 December 2019 by [email protected] https://academic.oup.com/biolinnean/article-abstract/128/4/865/5586699 Downloaded from other singing cicadas CHRIS SIMON1*, ERIC R. L. GORDON1, M. S. MOULDS2, JEFFREY A. COLE3, DILER HAJI1, ALAN R. LEMMON4, EMILY MORIARTY LEMMON5, MICHELLE KORTYNA5, KATHERINE NAZARIO1, ELIZABETH J. WADE1,6, RUSSELL C. MEISTER1, GEERT GOEMANS1, STEPHEN M. CHISWELL7, PABLO PESSACQ8, CLAUDIO VELOSO9, JOHN P. MCCUTCHEON10 and PIOTR ŁUKASIK10,11 1Department of Ecology & Evolutionary Biology, University of Connecticut, Storrs, CT 06268, USA 2Australian Museum Research Institute, Sydney, NSW 2010, Australia 3Natural Sciences Division, Pasadena City College, Pasadena, CA 91106, USA 4Department of Scientific Computing, Florida State University, Tallahassee, FL 32306–4120, USA 5Department of Biological Science, Florida State University, Tallahassee, FL 32306–4295, USA 6Department of Natural Sciences and Mathematics, Curry College, Milton, MA 02186, USA 7National Institute of Water and Atmosphere, Wellington, New Zealand 8Centro de Investigaciones Esquel de Montaña y Estepa Patagónicas, 9200 Esquel, Chubut, Argentina 9Department of Ecological Sciences, Science Faculty, University of Chile, 7800003 Santiago, Chile 10Division of Biological Sciences, University of Montana, Missoula, MT 59812, USA 11Department of Bioinformatics and Genetics, Swedish Museum of Natural History, 114 18 Stockholm, Sweden Received 11 May 2019; revised 13 July 2019; accepted for publication 14 July 2019 Phylogenetic asymmetry is common throughout the tree of life and results from contrasting patterns of speciation and extinction in the paired descendant lineages of ancestral nodes. -
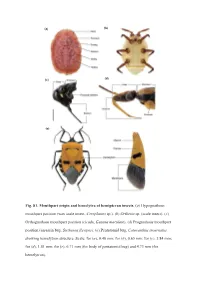
Page 1 (A) (E) Fig. S1. Mouthpart Origin and Hemelytra of Hemipteran
(a) (b) (d) (c) (e) Fig 61. Mouthpart origin and hemelytra of hemipteran insects. (a) Hypognathous mouthpart position (wax scale insect, Ceroplastes sp.). (b) Orthezia sp. (scale insect). (c) Orthognathous mouthpart position (cicada, Gaeana maculate). (d) Prognathous mouthpart position (assassin bug, Sirthenea flavipes). (e) Pentatomid bug, Catacanthus incarnatus showing hemelytron structure. Scale: for (a), 0.40 mm; for (b), 0.65 mm; for (c), 3.84 mm; for (d), 1.81 mm; for (e), 6.71 mm (for body of pentatomid bug) and 4.73 mm (for hemelytron). Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG1 PCG2 Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG3 RNA )LJ62. AliGROOVE analysis for codon positions of protein-coding genes (PCGs) and RNA genes. PCG1, the first codon position of PCGs. PCG2, the second codon position of PCGs. PCG3, the third codon position of PCGs. RNA, sequences of tRNA and rRNA genes. The mean similarity score between sequences is represented by a colored square, based on AliGROOVE scores from -1, indicating great difference in rates from the remainder of the data set, that is, heterogeneity (red coloring), to +1, indicating that ratesmatch all other comparisons (blue coloring). Bactericera sinica Sternorrhyncha Cicadomorpha Coleorrhyncha Fulgoromorpha Dipsocoromorpha Gerromorpha Enicocephalomorpha Nepomorpha Leptopodomorpha Cimicomorpha Heteroptera Pentatomomorpha Eusthenes cupreus FigS33K\ORJHQHWLFWUHHLQIHUUHGIURP3K\OR%D\HVDQDO\VLVRIWKH3&*51$GDWDVHWXQGHUWKe &$7*75PL[WXUHPRGHO9DOXHVDWQRGHVDUH%D\HVLDQ33V -

Bryophyte Ecology Table of Contents
Glime, J. M. 2020. Table of Contents. Bryophyte Ecology. Ebook sponsored by Michigan Technological University 1 and the International Association of Bryologists. Last updated 15 July 2020 and available at <https://digitalcommons.mtu.edu/bryophyte-ecology/>. This file will contain all the volumes, chapters, and headings within chapters to help you find what you want in the book. Once you enter a chapter, there will be a table of contents with clickable page numbers. To search the list, check the upper screen of your pdf reader for a search window or magnifying glass. If there is none, try Ctrl G to open one. TABLE OF CONTENTS BRYOPHYTE ECOLOGY VOLUME 1: PHYSIOLOGICAL ECOLOGY Chapter in Volume 1 1 INTRODUCTION Thinking on a New Scale Adaptations to Land Minimum Size Do Bryophytes Lack Diversity? The "Moss" What's in a Name? Phyla/Divisions Role of Bryology 2 LIFE CYCLES AND MORPHOLOGY 2-1: Meet the Bryophytes Definition of Bryophyte Nomenclature What Makes Bryophytes Unique Who are the Relatives? Two Branches Limitations of Scale Limited by Scale – and No Lignin Limited by Scale – Forced to Be Simple Limited by Scale – Needing to Swim Limited by Scale – and Housing an Embryo Higher Classifications and New Meanings New Meanings for the Term Bryophyte Differences within Bryobiotina 2-2: Life Cycles: Surviving Change The General Bryobiotina Life Cycle Dominant Generation The Life Cycle Life Cycle Controls Generation Time Importance Longevity and Totipotency 2-3: Marchantiophyta Distinguishing Marchantiophyta Elaters Leafy or Thallose? Class -

Karyotype of Karoophasma Biedouwense (Austrophasmatidae)
Eur. J. Entomol. 112(4): 599–605, 2015 doi: 10.14411/eje.2015.093 ISSN 1210-5759 (print), 1802-8829 (online) First chromosomal study of Mantophasmatodea: Karyotype of Karoophasma biedouwense (Austrophasmatidae) DOROTA LACHOWSKA-CIERLIK1, ANNA MARyAńSKA-Nadachowska2, VALENTINA KuZNETSOvA3 and MIKE PICKER4 1 Institute of Zoology, Jagiellonian university, Cracow, Poland; e-mail: [email protected] 2 Institute of Systematic and Evolution of Animals, PAS, Cracow, Poland; e-mail: [email protected] 3 Zoological Institute Russian Academy of Sciences, St. Petersburg, Russia; e-mail: [email protected] 4 Department of Biological Sciences, university of Cape Town, Rondebosch, Cape Town, South Africa; e-mail: [email protected] Key words. Mantophasmatodea, Karoophasma biedouwense, FISH, heterochromatin, heelwalkers, meiosis, ribosomal genes, sex determination system, telomeres Abstract. We have investigated for the first time the chromosomes of Karoophasma biedouwense, a species belonging to the Manto- phasmatodea, a recently discovered order of carnivorous insects. Our study has revealed that males of this species display testes with numerous seminal tubes (follicles), as in other Polyneoptera, and short tubular seminal vesicles embedded in a utricular gland. The karyotype consists of 2n = 12A + X monocentric and biarmed, meta/submetacentric chromosomes (fundamental number of arms: FN = 26) with blocks of heterochromatin around centromeres. The autosomes are classified into two size groups, one represented by a single, very large pair of autosomes, the other by five smaller pairs which constitute a continuous series gradually decreasing in size. Among “monocentric” orders of Polyneoptera, K. biedouwense shares its low chromosome number, 2n = 13, as also found with some Orthoptera (Acridoidea, Grylloidea, Gryllacridoidea). -

An Appraisal of the Higher Classification of Cicadas (Hemiptera: Cicadoidea) with Special Reference to the Australian Fauna
© Copyright Australian Museum, 2005 Records of the Australian Museum (2005) Vol. 57: 375–446. ISSN 0067-1975 An Appraisal of the Higher Classification of Cicadas (Hemiptera: Cicadoidea) with Special Reference to the Australian Fauna M.S. MOULDS Australian Museum, 6 College Street, Sydney NSW 2010, Australia [email protected] ABSTRACT. The history of cicada family classification is reviewed and the current status of all previously proposed families and subfamilies summarized. All tribal rankings associated with the Australian fauna are similarly documented. A cladistic analysis of generic relationships has been used to test the validity of currently held views on family and subfamily groupings. The analysis has been based upon an exhaustive study of nymphal and adult morphology, including both external and internal adult structures, and the first comparative study of male and female internal reproductive systems is included. Only two families are justified, the Tettigarctidae and Cicadidae. The latter are here considered to comprise three subfamilies, the Cicadinae, Cicadettinae n.stat. (= Tibicininae auct.) and the Tettigadinae (encompassing the Tibicinini, Platypediidae and Tettigadidae). Of particular note is the transfer of Tibicina Amyot, the type genus of the subfamily Tibicininae, to the subfamily Tettigadinae. The subfamily Plautillinae (containing only the genus Plautilla) is now placed at tribal rank within the Cicadinae. The subtribe Ydiellaria is raised to tribal rank. The American genus Magicicada Davis, previously of the tribe Tibicinini, now falls within the Taphurini. Three new tribes are recognized within the Australian fauna, the Tamasini n.tribe to accommodate Tamasa Distant and Parnkalla Distant, Jassopsaltriini n.tribe to accommodate Jassopsaltria Ashton and Burbungini n.tribe to accommodate Burbunga Distant.