Oral Candidosis: Pathophysiology and Best Practice for Diagnosis, Classification, and Successful Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
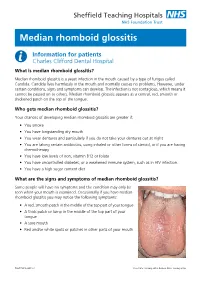
Median Rhomboid Glossitis
Median rhomboid glossitis Information for patients Charles Clifford Dental Hospital What is median rhomboid glossitis? Median rhomboid glossitis is a yeast infection in the mouth caused by a type of fungus called Candida. Candida lives harmlessly in the mouth and normally causes no problems. However, under certain conditions, signs and symptoms can develop. The infection is not contagious, which means it cannot be passed on to others. Median rhomboid glossitis appears as a central, red, smooth or thickened patch on the top of the tongue. Who gets median rhomboid glossitis? Your chances of developing median rhomboid glossitis are greater if: • You smoke • You have longstanding dry mouth • You wear dentures and particularly if you do not take your dentures out at night • You are taking certain antibiotics, using inhaled or other forms of steroid, or if you are having chemotherapy • You have low levels of iron, vitamin B12 or folate • You have uncontrolled diabetes, or a weakened immune system, such as in HIV infection. • You have a high sugar content diet What are the signs and symptoms of median rhomboid glossitis? Some people will have no symptoms and the condition may only be seen when your mouth is examined. Occasionally if you have median rhomboid glossitis you may notice the following symptoms: • A red, smooth patch in the middle of the top part of your tongue • A thick patch or lump in the middle of the top part of your tongue • A sore mouth • Red and/or white spots or patches in other parts of your mouth PD6779-PIL2645 v4 Issue Date: January 2019. -

Zeroing in on the Cause of Your Patient's Facial Pain
Feras Ghazal, DDS; Mohammed Ahmad, Zeroing in on the cause MD; Hussein Elrawy, DDS; Tamer Said, MD Department of Oral Health of your patient's facial pain (Drs. Ghazal and Elrawy) and Department of Family Medicine/Geriatrics (Drs. Ahmad and Said), The overlapping characteristics of facial pain can make it MetroHealth Medical Center, Cleveland, Ohio difficult to pinpoint the cause. This article, with a handy at-a-glance table, can help. [email protected] The authors reported no potential conflict of interest relevant to this article. acial pain is a common complaint: Up to 22% of adults PracticE in the United States experience orofacial pain during recommendationS F any 6-month period.1 Yet this type of pain can be dif- › Advise patients who have a ficult to diagnose due to the many structures of the face and temporomandibular mouth, pain referral patterns, and insufficient diagnostic tools. disorder that in addition to Specifically, extraoral facial pain can be the result of tem- taking their medication as poromandibular disorders, neuropathic disorders, vascular prescribed, they should limit disorders, or atypical causes, whereas facial pain stemming activities that require moving their jaw, modify their diet, from inside the mouth can have a dental or nondental cause and minimize stress; they (FIGURE). Overlapping characteristics can make it difficult to may require physical therapy distinguish these disorders. To help you to better diagnose and and therapeutic exercises. C manage facial pain, we describe the most common causes and underlying pathological processes. › Consider prescribing a tricyclic antidepressant for patients with persistent idiopathic facial pain. C Extraoral facial pain Extraoral pain refers to the pain that occurs on the face out- 2-15 Strength of recommendation (SoR) side of the oral cavity. -

Cutaneous Manifestations of HIV Infection Carrie L
Chapter Title Cutaneous Manifestations of HIV Infection Carrie L. Kovarik, MD Addy Kekitiinwa, MB, ChB Heidi Schwarzwald, MD, MPH Objectives Table 1. Cutaneous manifestations of HIV 1. Review the most common cutaneous Cause Manifestations manifestations of human immunodeficiency Neoplasia Kaposi sarcoma virus (HIV) infection. Lymphoma 2. Describe the methods of diagnosis and treatment Squamous cell carcinoma for each cutaneous disease. Infectious Herpes zoster Herpes simplex virus infections Superficial fungal infections Key Points Angular cheilitis 1. Cutaneous lesions are often the first Chancroid manifestation of HIV noted by patients and Cryptococcus Histoplasmosis health professionals. Human papillomavirus (verruca vulgaris, 2. Cutaneous lesions occur frequently in both adults verruca plana, condyloma) and children infected with HIV. Impetigo 3. Diagnosis of several mucocutaneous diseases Lymphogranuloma venereum in the setting of HIV will allow appropriate Molluscum contagiosum treatment and prevention of complications. Syphilis Furunculosis 4. Prompt diagnosis and treatment of cutaneous Folliculitis manifestations can prevent complications and Pyomyositis improve quality of life for HIV-infected persons. Other Pruritic papular eruption Seborrheic dermatitis Overview Drug eruption Vasculitis Many people with human immunodeficiency virus Psoriasis (HIV) infection develop cutaneous lesions. The risk of Hyperpigmentation developing cutaneous manifestations increases with Photodermatitis disease progression. As immunosuppression increases, Atopic Dermatitis patients may develop multiple skin diseases at once, Hair changes atypical-appearing skin lesions, or diseases that are refractory to standard treatment. Skin conditions that have been associated with HIV infection are listed in Clinical staging is useful in the initial assessment of a Table 1. patient, at the time the patient enters into long-term HIV care, and for monitoring a patient’s disease progression. -

Metronidazole Induced Aphthous Ulcer with Angular Cheilitis
Pharmacy & Pharmacology International Journal Case Report Open Access Metronidazole induced aphthous ulcer with angular cheilitis Abstract Volume 4 Issue 3 - 2016 Metronidazole is an antiprotozoal drug, which has broad spectrum cidal activity Aruna Bhushan,1 Ved Bhushan ST2 against anaerobic protozoa and microaerophillic bacteria. Aphthous ulcer is a very 1Associate Professor, Department of Pharmacology, India rare side effect with metronidazole. Here we report a case of 55 year old male suffered 2Professor of Surgery, KLE- Centrinary Charitable Hospital, from metronidazole induced aphthous ulcer with angular cheilitis. India metronidazole, adrs, cheilites Keywords: Correspondence: Aruna Bhushan, Associate Professor, Department of Pharmacology, BIMS, Karnataka, India, Tel 9480538661, Email [email protected] Received: April 04, 2016 | Published: April 19, 2016 Introduction complex and Anti histaminic CPM (chlorpheniramine maleate 10mg tablets) twice daily was started. Patient was also prescribed topical Metronidazole, chemically a nitroimidazole is an antiprotozoal anesthetics Zytee (choline salicylate and benzalkonium chloride drug, which has broad spectrum cidal activity against anaerobic solution 10ml gel) small quantity to be applied on affected area twice protozoa, anaerobic and microaerophillic bacteria. It was introduced daily. in 1959 for trichomoniasis, and later found to be highly active amoebicide. Metronidazole after entering the cell by diffusion, its The patient gradually and progressively improved within 5-7days nitro group is reduced by certain redox proteins to a highly reactive lesions resolved within 7-10days and completely recovered in 2weeks. nitro radical, which acts as an electron sink competes with the biological electron acceptors generated by cell mitochondria and Discussion hence interferes with energy metabolism. The drug is completely Metronidazole is a frequently prescribed drug for amoebiasis, absorbed orally, metabolized in liver followed by renal excretion. -

Oral Candidosis: Aetiology, Clinical Manifestations, Diagnosis and Management Birsay Gümrü Tarçın
MÜSBED 2011;1(2):140-148 Derleme / Review Oral Candidosis: Aetiology, Clinical Manifestations, Diagnosis and Management Birsay Gümrü Tarçın Marmara University Faculty of Dentistry, Department of Oral Diagnosis and Radiology, Istanbul-Turkey Ya zış ma Ad re si / Add ress rep rint re qu ests to: Birsay Gümrü Tarçın Marmara University Faculty of Dentistry, Department of Oral Diagnosis and Radiology, Büyükçiftlik Sok. No: 6 34365 Nişantaşı, Şişli, İstanbul-Turkey Telefon / Phone: +90-212-231-9120 Faks / Fax: +90-212-246-5247 Elekt ro nik pos ta ad re si / E-ma il add ress: [email protected] Ka bul ta ri hi / Da te of ac cep tan ce: 22 Ağustos 2011 / August 22, 2011 ÖZET ABS TRACT Oral kandidozis: Etiyoloji, klinik özellikler, tanı Oral candidosis: aetiology, clinical ve tedavi manifestations, diagnosis and management Oral kandidozis dişhekimliği pratiğinde en sık karşılaşılan fungal Oral candidosis is the most common fungal infection encountered enfeksiyondur. Birçok farklı klinik görünümde ortaya çıkabildi- in dental practice. Clinical diagnosis and management of oral ğinden, klinik tanı ve tedavisi genellikle zordur. Hastalık sıklıkla candidosis is usually complicated, because it is encountered in a sistemik rahatsızlığı olan spesifik hasta gruplarında ortaya çık- wide variety of clinical presentations. The disease often manifests maktadır. Bu nedenle, oral kandidozis tedavisi öncelikle predis- in specific patient groups that are systemically compromised. pozisyon yaratan durumların kapsamlı tetkikini içermelidir. Bu Therefore, the management should always cover a thorough derlemede sık karşılaşılan oral kandidal lezyonların etiyolojisi, investigation of underlying predisposing conditions. This klinik görünümü, tanı ve tedavi stratejileri kapsamlı bir şekilde review provides a comprehensive overview of the aetiology, gözden geçirilmektedir. -

Alcohol Use and Oral Health Fact Sheet for PROVIDERS OCTOBER 2017
Alcohol Use and Oral Health Fact Sheet FOR PROVIDERS OCTOBER 2017 The Challenge… Glossitis – tongue inflammation Patients who drink alcohol regularly may experience specific problems related to their oral health and hygiene. Angular cheilitis – corners of the mouth chronically inflamed and cracked What you need to know… Candida – yeast infection • Patients who drink high amounts of alcohol daily may brush Oral Ulceration – painful round or oval less effectively than those who don’t drink alcohol, despite sores reporting similar brushing frequency. Also, impaired motor Acute Necrotizing activity can affect their ability to perform basic dental hygiene adequately.1 Ulcerative Gingivitis – infection of the gums that causes ulcers, swelling, and • Alcohol is also the most common cause of sialadenosis dead tissue in the mouth of the parotid gland. This condition causes swelling of the parotid gland and decreased secretion of saliva.2 Ways You Can Help… • Poor nutrient intake and absorption combined with decreased salivary excretion frequently can lead to glossitis, Recommend: angular cheilitis, candida infection, oral ulceration, and acute • Brushing thoroughly two times daily with a necrotizing ulcerative gingivitis (ANUG).2 fluoridated toothpaste. • A decreased immune response combined with a nutritionally • Rinse mouth with non-alcoholic mouth rinse. poor diet, poor oral hygiene, decreased salivary flow, and a • Have an oral examination and cleaning by a high incidence of smoking among these patients, provides dental professional at least two times per year. an environment conducive to rapid progression of periodontal • Regular oral exams that include a periodontal disease, dental caries and increased risk of oral thoracic evaluation and oral cancer screenings to detect cancers.2 any signs of suspicious lesions.3 • High consumption of alcohol may damage the liver and bone marrow resulting in excessive bleeding during dental treatment. -

Cardiovascular Drugs-Induced Oral Toxicities: a Murky Area to Be Revisited and Illuminated
Pharmacological Research 102 (2015) 81–89 Contents lists available at ScienceDirect Pharmacological Research j ournal homepage: www.elsevier.com/locate/yphrs Review Cardiovascular drugs-induced oral toxicities: A murky area to be revisited and illuminated a, b b Pitchai Balakumar ∗, Muthu Kavitha , Suresh Nanditha a Pharmacology Unit, Faculty of Pharmacy, AIMST University, Semeling, 08100 Bedong, Malaysia b Faculty of Dentistry, AIMST University, 08100 Bedong, Malaysia a r t i c l e i n f o a b s t r a c t Article history: Oral health is an imperative part of overall human health. Oral disorders are often unreported, but are Received 20 July 2015 highly troublesome to human health in a long-standing situation. A strong association exists between Received in revised form 22 August 2015 cardiovascular drugs and oral adverse effects. Indeed, several cardiovascular drugs employed clinically Accepted 8 September 2015 have been reported to cause oral adverse effects such as xerostomia, oral lichen planus, angioedema, Available online 25 September 2015 aphthae, dysgeusia, gingival enlargement, scalded mouth syndrome, cheilitis, glossitis and so forth. Oral complications might in turn worsen the cardiovascular disease condition as some reports suggest an Keywords: adverse correlation between periodontal oral disease pathogenesis and cardiovascular disease. These are Cardiovascular drugs certainly important to be understood for a better use of cardiovascular medicines and control of associated Oral adverse effects oral adverse effects. This review sheds lights on the oral adverse effects pertaining to the clinical use of Dry mouth Angioedema cardiovascular drugs. Above and beyond, an adverse correlation between oral disease and cardiovascular Dysgeusia disease has been discussed. -

HIV Infection and AIDS
G Maartens 12 HIV infection and AIDS Clinical examination in HIV disease 306 Prevention of opportunistic infections 323 Epidemiology 308 Preventing exposure 323 Global and regional epidemics 308 Chemoprophylaxis 323 Modes of transmission 308 Immunisation 324 Virology and immunology 309 Antiretroviral therapy 324 ART complications 325 Diagnosis and investigations 310 ART in special situations 326 Diagnosing HIV infection 310 Prevention of HIV 327 Viral load and CD4 counts 311 Clinical manifestations of HIV 311 Presenting problems in HIV infection 312 Lymphadenopathy 313 Weight loss 313 Fever 313 Mucocutaneous disease 314 Gastrointestinal disease 316 Hepatobiliary disease 317 Respiratory disease 318 Nervous system and eye disease 319 Rheumatological disease 321 Haematological abnormalities 322 Renal disease 322 Cardiac disease 322 HIV-related cancers 322 306 • HIV INFECTION AND AIDS Clinical examination in HIV disease 2 Oropharynx 34Neck Eyes Mucous membranes Lymph node enlargement Retina Tuberculosis Toxoplasmosis Lymphoma HIV retinopathy Kaposi’s sarcoma Progressive outer retinal Persistent generalised necrosis lymphadenopathy Parotidomegaly Oropharyngeal candidiasis Cytomegalovirus retinitis Cervical lymphadenopathy 3 Oral hairy leucoplakia 5 Central nervous system Herpes simplex Higher mental function Aphthous ulcers 4 HIV dementia Kaposi’s sarcoma Progressive multifocal leucoencephalopathy Teeth Focal signs 5 Toxoplasmosis Primary CNS lymphoma Neck stiffness Cryptococcal meningitis 2 Tuberculous meningitis Pneumococcal meningitis 6 -

Adverse Effects of Medications on Oral Health
Adverse Effects of Medications on Oral Health Dr. James Krebs, BS Pharm, MS, PharmD Director of Experiential Education College of Pharmacy, University of New England Presented by: Rachel Foster PharmD Candidate, Class of 2014 University of New England October 2013 Objectives • Describe the pathophysiology of various medication-related oral reactions • Recognize the signs and symptoms associated with medication-related oral reactions • Identify the populations associated with various offending agents • Compare the treatment options for medication-related oral reactions Medication-related Oral Reactions • Stomatitis • Oral Candidiasis • Burning mouth • Gingival hyperplasia syndrome • Alterations in • Glossitis salivation • Erythema • Alterations in taste Multiforme • Halitosis • Oral pigmentation • Angioedema • Tooth discoloration • Black hairy tongue Medication-related Stomatitis • Clinical presentation – Aphthous-like ulcers, mucositis, fixed-drug eruption, lichen planus1,2 – Open sores in the mouth • Tongue, gum line, buccal membrane – Patient complaint of soreness or burning http://www.virtualmedicalcentre.com/diseases/oral-mucositis-om/92 0 http://www.virtualmedicalcentre.com/diseases/oral-mucositis-om/920 Medication-related Stomatitis • Offending agents1,2 Medication Indication Patient Population Aspirin •Heart health • >18 years old •Pain reliever • Cardiac patients NSAIDs (i.e. Ibuprofen, •Headache General population naproxen) •Pain reliever •Fever reducer Chemotherapy (i.e. •Breast cancer •Oncology patients methotrexate, 5FU, •Colon -

Oral Manifestations of Pemphigus Vulgaris
Journal of Clinical & Experimental Dermatology Research - Open Access Research Article OPEN ACCESS Freely available online doi:10.4172/2155-9554.1000112 Oral Manifestations of Pemphigus Vulgaris: Clinical Presentation, Differential Diagnosis and Management Antonio Bascones-Martinez1*, Marta Munoz-Corcuera2, Cristina Bascones-Ilundain1 and German Esparza-Gómez1 1DDS, PhD, Medicine and Bucofacial Surgery Department, Dental School, Complutense University of Madrid, Spain 2DDS, PhD Student, Medicine and Bucofacial Surgery Department, Dental School, Complutense University of Madrid, Spain Abstract Pemphigus vulgaris is a chronic autoimmune mucocutaneous disease characterized by the formation of intraepithelial blisters. It results from an autoimmune process in which antibodies are produced against desmoglein 1 and desmoglein 3, normal components of the cell membrane of keratinocytes. The first manifestations of pemphigus vulgaris appear in the oral mucosa in the majority of patients, followed at a later date by cutaneous lesions. The diagnosis is based on clinical findings and laboratory analyses, and it is usually treated by the combined administration of corticosteroids and immunosuppressants. Detection of the oral lesions can result in an earlier diagnosis. We review the oral manifestations of pemphigus vulgaris as well as the differential diagnosis, treatment, and prognosis of oral lesions in this uncommon disease. Keywords: Pemphigus; Oral mucosa; Autoimmune bullous disease and have a molecular weight of 130 and 160 KDa, respectively [1,7,9,13]. The binding of antibodies to desmoglein at mucosal or Introduction cutaneous level gives rise to the loss of cell adhesion, with separation of epithelial layers (acantholysis) and the consequent appearance of Pemphigus vulgaris (PV) is the most frequently observed blisters on skin or mucosae [1,3]. -
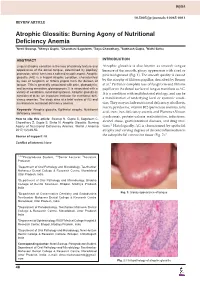
Atrophic Glossitis: Burning Agony of Nutritional Deficiency Anemia 1Neeti Swarup, 2Shreya Gupta, 3Chandrani Sagolsem, 4Zoya Chowdhary, 5Subhash Gupta, 6Nidhi Sinha
WJOA Neeti Swarup et al 10.5005/jp-journals-10065-0011 REVIEW ARTICLE Atrophic Glossitis: Burning Agony of Nutritional Deficiency Anemia 1Neeti Swarup, 2Shreya Gupta, 3Chandrani Sagolsem, 4Zoya Chowdhary, 5Subhash Gupta, 6Nidhi Sinha ABSTRACT INTRODUCTION Lingual atrophic condition is the loss of ordinary texture and Atrophic glossitis is also known as smooth tongue appearance of the dorsal tongue, determined by papillary because of the smooth, glossy appearance with a red or protrusion, which turns into a soft and smooth aspect. Atrophic pink background (Fig. 1). The smooth quality is caused glossitis (AG) is a lingual atrophic condition, characterized by loss of fungiform or filiform papilla from the dorsum of by the atrophy of filiform papillae, described by Reamy 1 tongue. This is generally associated with pain, glossodynia, et al. Partial or complete loss of fungiform and filiform and burning sensation, glossopyrosis. It is associated with a papillae on the dorsal surface of tongue manifests as AG. variety of conditions, local and systemic. Atrophic glossitis is It is a condition with multifactorial etiology, and can be considered to be an important indicator for nutritional defi- ciency anemias. The study aims at a brief review of AG and a manifestation of underlying local or systemic condi- its relation to nutritional deficiency anemia. tion. They may include nutritional deficiency, riboflavin, niacin, pyridoxine, vitamin B12 (pernicious anemia), folic Keywords: Atrophic glossitis, Epithelial atrophy, Nutritional deficiency anemia. acid, iron (iron deficiency anemia and Plummer-Vinson syndrome), protein-calorie malnutrition, infections, How to cite this article: Swarup N, Gupta S, Sagolsem C, alcohol abuse, gastrointestinal diseases, and drug reac- Chowdhary Z, Gupta S, Sinha N. -

Giant Cell Arteritis Misdiagnosed As Temporomandibular Disorder: a Case Report and Review of the Literature
360_Reiter.qxp 10/14/09 3:17 PM Page 360 Giant Cell Arteritis Misdiagnosed as Temporomandibular Disorder: A Case Report and Review of the Literature Shoshana Reiter, DMD Giant cell arteritis (GCA) is a systemic vasculitis involving medium Teacher and large-sized arteries, most commonly the extracranial branches Department of Oral Rehabilitation of the carotid artery. Early diagnosis and treatment are essential to avoid severe complications. This article reports on a GCA case Ephraim Winocur, DMD and discusses how the orofacial manifestations of GCA can lead to Lecturer misdiagnosis of GCA as temporomandibular disorder. GCA Department of Oral Rehabilitation should be included in the differential diagnosis of orofacial pain in Carole Goldsmith, DMD the elderly based on the knowledge of related signs and symptoms, Instructor mainly jaw claudication, hard end-feel limitation of range of Department of Oral Rehabilitation motion, and temporal headache. J OROFAC PAIN 2009;23:360–365 Alona Emodi-Perlman, DMD Key words: Giant cell arteritis, jaw claudication, Teacher temporomandibular disorders, trismus Department of Oral Rehabilitation Meir Gorsky, DMD Professor Department of Oral Pathology and Oral iant cell arteritis (GCA) is a systemic vasculitis involving Medicine the large and medium-sized vessels, particularly the extracranial branches of the carotid artery. It is more com- The Maurice and Gabriela Goldschleger G School of Dental Medicine mon in women (M:F ratio 2:5) and usually affects patients older 1 Tel Aviv University, Israel than 50 years with an increased risk with age. The highest preva- lence of GCA has been reported in Scandinavian populations and Correspondence to: in those with a strong Scandinavian ethnic background.2 Dr.