A Kingdom in Decline: Holocene Range Contraction of the Lion (Panthera Leo) Modelled with Global Environmental Stratification
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Syntactic Changes in English Between the Seventeenth Century and The
I Syntactic Changes in English between the Seventeenth Century and the Twentieth Century as Represented in Two Literary Works: William Shakespeare's Play The Merchant of Venice and George Bernard Shaw's Play Arms and the Man التغيرات النحوية في اللغة اﻹنجليزية بين القرن السابع عشر و القرن العشرين ممثلة في عملين أدبيين : مسرحية تاجر البندقيه لوليام شكسبيرو مسرحية الرجل والسﻻح لجورج برنارد شو By Eman Mahmud Ayesh El-Abweni Supervised by Prof. Zakaria Ahmad Abuhamdia A Thesis Submitted in Partial Fulfillment of the Requirements for the Master’s Degree in English Language and Literature Department of English Language and Literature Faculty of Arts and Sciences Middle East University January, 2018 II III IV Acknowledgments First and above all, the whole thanks and glory are for the Almighty Allah with His Mercy, who gave me the strength and fortitude to finish my thesis. I would like to express my trustworthy gratitude and appreciation for my supervisor Professor Zakaria Ahmad Abuhamdia for his unlimited guidance and supervision. I have been extremely proud to have a supervisor who appreciated my work and responded to my questions either face- to- face, via the phone calls or, SMS. Without his support my thesis, may not have been completed successfully. Also, I would like to thank the committee members for their comments and guidance. My deepest and great gratitude is due to my parents Mahmoud El-Abweni and Intisar El-Amayreh and my husband Amjad El-Amayreh who have supported and encouraged me to reach this stage. In addition, my appreciation is extended to my brothers Ayesh, Yousef and my sisters Saja and Noor for their support and care during this period, in addition to my beloved children Mohammad and Aded El-Rahman who have been a delight. -

Slow-Onset Processes and Resulting Loss and Damage
Publication Series ADDRESSING LOSS AND DAMAGE FROM SLOW-ONSET PROCESSES Slow-onset Processes and Resulting Loss and Damage – An introduction Table of contents L 4 22 ist of a bbre Summary of Loss and damage via tio key facts and due to slow-onset ns definitions processes AR4 IPCC Fourth Assessment Report 6 22 What is loss and damage? Introduction AR5 IPCC Fifth Assessment Report COP Conference of the Parties to the 23 United Nations Framework Convention on 9 What losses and damages IMPRINT Climate Change can result from slow-onset Slow-onset ENDA Environment Development Action Energy, processes? Authors Environment and Development Programme processes and their Laura Schäfer, Pia Jorks, Emmanuel Seck, Energy key characteristics 26 Oumou Koulibaly, Aliou Diouf ESL Extreme Sea Level What losses and damages Contributors GDP Gross Domestic Product 9 can result from sea level rise? Idy Niang, Bounama Dieye, Omar Sow, Vera GMSL Global mean sea level What is a slow-onset process? Künzel, Rixa Schwarz, Erin Roberts, Roxana 31 Baldrich, Nathalie Koffi Nguessan GMSLR Global mean sea level rise 10 IOM International Organization on Migration What are key characteristics Loss and damage Editing Adam Goulston – Scize Group LLC of slow-onset processes? in Senegal due to IPCC Intergovernmental Panel on Climate Change sea level rise Layout and graphics LECZ Low-elevation coastal zone 14 Karin Roth – Wissen in Worten OCHA Office for the Coordination of Humanitarian Affairs What are other relevant January 2021 terms for the terminology on 35 RCP Representative -

Desertification and Agriculture
BRIEFING Desertification and agriculture SUMMARY Desertification is a land degradation process that occurs in drylands. It affects the land's capacity to supply ecosystem services, such as producing food or hosting biodiversity, to mention the most well-known ones. Its drivers are related to both human activity and the climate, and depend on the specific context. More than 1 billion people in some 100 countries face some level of risk related to the effects of desertification. Climate change can further increase the risk of desertification for those regions of the world that may change into drylands for climatic reasons. Desertification is reversible, but that requires proper indicators to send out alerts about the potential risk of desertification while there is still time and scope for remedial action. However, issues related to the availability and comparability of data across various regions of the world pose big challenges when it comes to measuring and monitoring desertification processes. The United Nations Convention to Combat Desertification and the UN sustainable development goals provide a global framework for assessing desertification. The 2018 World Atlas of Desertification introduced the concept of 'convergence of evidence' to identify areas where multiple pressures cause land change processes relevant to land degradation, of which desertification is a striking example. Desertification involves many environmental and socio-economic aspects. It has many causes and triggers many consequences. A major cause is unsustainable agriculture, a major consequence is the threat to food production. To fully comprehend this two-way relationship requires to understand how agriculture affects land quality, what risks land degradation poses for agricultural production and to what extent a change in agricultural practices can reverse the trend. -
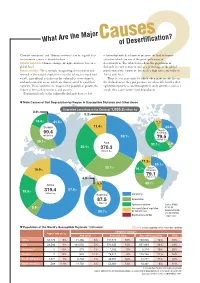
What Are the Major Causes of Desertification?
What Are the Major Causesof Desertification? ‘Climatic variations’ and ‘Human activities’ can be regarded as relationship with development pressure on land by human the two main causes of desertification. activities which are one of the principal causes of Climatic variations: Climate change, drought, moisture loss on a desertification. The table below shows the population in global level drylands by each continent and as a percentage of the global Human activities: These include overgrazing, deforestation and population of the continent. It reveals a high ratio especially in removal of the natural vegetation cover(by taking too much fuel Africa and Asia. wood), agricultural activities in the vulnerable ecosystems of There is a vicious circle by which when many people live in arid and semi-arid areas, which are thus strained beyond their the dryland areas, they put pressure on vulnerable land by their capacity. These activities are triggered by population growth, the agricultural practices and through their daily activities, and as a impact of the market economy, and poverty. result, they cause further land degradation. Population levels of the vulnerable drylands have a close 2 ▼ Main Causes of Soil Degradation by Region in Susceptible Drylands and Other Areas Degraded Land Area in the Dryland: 1,035.2 million ha 0.9% 0.3% 18.4% 41.5% 7.7 % Europe 11.4% 34.8% North 99.4 America million ha 32.1% 79.5 million ha 39.1% Asia 52.1% 5.4 26.1% 370.3 % million ha 11.5% 33.1% 30.1% South 16.9% 14.7% America 79.1 million ha 4.8% 5.5 40.7% Africa -

Assessing Climate Change's Contribution to Global Catastrophic
Assessing Climate Change’s Contribution to Global Catastrophic Risk Simon Beard,1,2 Lauren Holt,1 Shahar Avin,1 Asaf Tzachor,1 Luke Kemp,1,3 Seán Ó hÉigeartaigh,1,4 Phil Torres, and Haydn Belfield1 5 A growing number of people and organizations have claimed climate change is an imminent threat to human civilization and survival but there is currently no way to verify such claims. This paper considers what is already known about this risk and describes new ways of assessing it. First, it reviews existing assessments of climate change’s contribution to global catastrophic risk and their limitations. It then introduces new conceptual and evaluative tools, being developed by scholars of global catastrophic risk that could help to overcome these limitations. These connect global catastrophic risk to planetary boundary concepts, classify its key features, and place global catastrophes in a broader policy context. While not yet constituting a comprehensive risk assessment; applying these tools can yield new insights and suggest plausible models of how climate change could cause a global catastrophe. Climate Change; Global Catastrophic Risk; Planetary Boundaries; Food Security; Conflict “Understanding the long-term consequences of nuclear war is not a problem amenable to experimental verification – at least not more than once" Carl Sagan (1983) With these words, Carl Sagan opened one of the most influential papers ever written on the possibility of a global catastrophe. “Nuclear war and climatic catastrophe: Some policy implications” set out a clear and credible mechanism by which nuclear war might lead to human extinction or global civilization collapse by triggering a nuclear winter. -

History Through the Ages (Solucionario) 3 SOLUTIONS
Material AICLE. 5º de Primaria.: History Through the Ages (Solucionario) 3 SOLUTIONS Activity 2. Read and complete the chart Historical sources Oral Written Graphic Materials - Paintings - Ruins - Books - Songs - Maps - Monuments - Theatre plays - Photos - Graves - Poems - Sayings - Films - Coins - Letters - Newspappers Activity nº 3. Match the historical sources with the related words and the images 4 Material AICLE. 5º de Primaria.: History Through the Ages (Solucionario) Activity nº 4. Read the timeline carefully. Then listen and complete the text below AGES OF HISTORY History is divided into five different ages: Prehistory, Ancient History, the Middle Ages, the Modern Age and the Contemporary Age. PREHISTORY extended from the time the first human beings appeared until the invention of writing. ANCIENT HISTORY extended from the invention of writing until the fall of the Roman Empire. The MIDDLE AGES extended from the fall of the Roman Empire until the discovery of the Americas. The MODERN AGE extended from the discovery of the Americas until the French Revolution. The CONTEMPORARY AGE extended from the French Revolution until present day. Activity nº 6. Read the following text about Prehistory. Then choose the correct answers 1. Prehistory is divided in: Two periods: the Palaeolithic Age and the Neolithic Age. 2. The Palaeolithic Age extended: From the time the first human beings appeared until the development of livestock farming and agriculture. 3. During the Palaeolithic Age people were: Nomads. 4. During the Palaeolithic Age people: Hunted, fished and picked wild berries. 5. During the Palaeolithic Age: They have tools made of stone, wood, bones or shells. 6. -

Re-Thinking Policies to Cope with Desertification
Overcoming One of the Greatest Environmental Challenges of Our Times: Re-thinking Policies to Cope with Desertification Authors: Zafar Adeel, Janos Bogardi, Christopher Braeuel, Pamela Chasek, Maryam Niamir-Fuller, Donald Gabriels, Caroline King, Friederike Knabe, Ahang Kowsar, Boshra Salem, Thomas Schaaf, Gemma Shepherd, and Richard Thomas Overcoming One of the Greatest Environmental Challenges of Our Times: Re-thinking Policies to Cope with Desertification A Policy Brief based on The Joint International Conference: “Desertification and the International Policy Imperative” Algiers, Algeria, 17-19 December, 2006 Authors: Zafar Adeel, Janos Bogardi, Christopher Braeuel, Pamela Chasek, Maryam Niamir-Fuller, Donald Gabriels, Caroline King, Friederike Knabe, Ahang Kowsar, Boshra Salem, Thomas Schaaf, Gemma Shepherd, and Richard Thomas Partners: Algerian Ministry of Land Planning and Environment Foreword ver the past few dwindling interest in addressing this issue as a Oyears, it has become full-blown global challenge. Policies, whether increasingly clear that implemented at the national or international level, desertification is one of are failing to take full account of this slow, creeping the most pressing global problem when addressing poverty and economic environmental challenges development at large. Some forces of globalization, of our time, threatening while striving to reduce economic inequality and to reverse the gains in eliminate poverty, are in actuality contributing to the sustainable development worsening desertification. Perverse agricultural subsidies that we have seen emerge are one such example. in many parts of the world. It is a process that UNU has a mission to bridge the divide between can inherently destabilize the research and policy-making communities in societies by deepening order to address pressing global challenges such as poverty and creating environmental refugees who can desertification. -
The History of Prehistoric Research in Indonesia to 1950
The History of Prehistoric Research in Indonesia to 1950 Received 16 January 1968 R. P. SOE]ONO INTRODUCTION HE oldest description of material valuable for prehistoric recording in future times was given by G. E. Rumphius at the beginning of the eighteenth century. Rum T phius mentioned the veneration of historical objects by local peoples, and even now survivals of the very remote past retain their respect. On several islands we also notice a continuation of prehistoric traditions and art. Specific prehistoric relics, like many other archaeological remains, are holy to most of the inhabitants because of their quaint, uncommon shapes. As a result, myths are frequently created around these objects. An investigator is not permitted to inspect the bronze kettle drum kept in a temple at Pedjeng (Bali) and he must respect the people's devout feelings when he attempts to observe megalithic relics in the Pasemah Plateau (South Sumatra); these facts accentuate the persistence oflocal veneration even today. In spite of the veneration of particular objects, which in turn favors their preservation, many other relics have been lost or destroyed through digging or looting by treasure hunters or other exploiters seeking economic gain. Moreover, unqualified excavators have com pounded the problem. P. V. van Stein Callenfels, originally a specialist in Hindu-Indonesian archaeology, be came strongly aware of neglect in the field of prehistoric archaeology, and he took steps to begin systematic research. During his visit to kitchen middens in East Sumatra during a tour of inspection in 1920 for the Archaeological Service, he met with the digging of shell heaps for shell for limekilns. -

Download Global Catastrophic Risks 2020
Global Catastrophic Risks 2020 Global Catastrophic Risks 2020 INTRODUCTION GLOBAL CHALLENGES FOUNDATION (GCF) ANNUAL REPORT: GCF & THOUGHT LEADERS SHARING WHAT YOU NEED TO KNOW ON GLOBAL CATASTROPHIC RISKS 2020 The views expressed in this report are those of the authors. Their statements are not necessarily endorsed by the affiliated organisations or the Global Challenges Foundation. ANNUAL REPORT TEAM Ulrika Westin, editor-in-chief Waldemar Ingdahl, researcher Victoria Wariaro, coordinator Weber Shandwick, creative director and graphic design. CONTRIBUTORS Kennette Benedict Senior Advisor, Bulletin of Atomic Scientists Angela Kane Senior Fellow, Vienna Centre for Disarmament and Non-Proliferation; visiting Professor, Sciences Po Paris; former High Representative for Disarmament Affairs at the United Nations Joana Castro Pereira Postdoctoral Researcher at Portuguese Institute of International Relations, NOVA University of Lisbon Philip Osano Research Fellow, Natural Resources and Ecosystems, Stockholm Environment Institute David Heymann Head and Senior Fellow, Centre on Global Health Security, Chatham House, Professor of Infectious Disease Epidemiology, London School of Hygiene & Tropical Medicine Romana Kofler, United Nations Office for Outer Space Affairs Lindley Johnson, NASA Planetary Defense Officer and Program Executive of the Planetary Defense Coordination Office Gerhard Drolshagen, University of Oldenburg and the European Space Agency Stephen Sparks Professor, School of Earth Sciences, University of Bristol Ariel Conn Founder and -

A STUDY of WRITING Oi.Uchicago.Edu Oi.Uchicago.Edu /MAAM^MA
oi.uchicago.edu A STUDY OF WRITING oi.uchicago.edu oi.uchicago.edu /MAAM^MA. A STUDY OF "*?• ,fii WRITING REVISED EDITION I. J. GELB Phoenix Books THE UNIVERSITY OF CHICAGO PRESS oi.uchicago.edu This book is also available in a clothbound edition from THE UNIVERSITY OF CHICAGO PRESS TO THE MOKSTADS THE UNIVERSITY OF CHICAGO PRESS, CHICAGO & LONDON The University of Toronto Press, Toronto 5, Canada Copyright 1952 in the International Copyright Union. All rights reserved. Published 1952. Second Edition 1963. First Phoenix Impression 1963. Printed in the United States of America oi.uchicago.edu PREFACE HE book contains twelve chapters, but it can be broken up structurally into five parts. First, the place of writing among the various systems of human inter communication is discussed. This is followed by four Tchapters devoted to the descriptive and comparative treatment of the various types of writing in the world. The sixth chapter deals with the evolution of writing from the earliest stages of picture writing to a full alphabet. The next four chapters deal with general problems, such as the future of writing and the relationship of writing to speech, art, and religion. Of the two final chapters, one contains the first attempt to establish a full terminology of writing, the other an extensive bibliography. The aim of this study is to lay a foundation for a new science of writing which might be called grammatology. While the general histories of writing treat individual writings mainly from a descriptive-historical point of view, the new science attempts to establish general principles governing the use and evolution of writing on a comparative-typological basis. -

Modernity: Religious Trends
Universal Rights in a World of Diversity. The Case of Religious Freedom Pontifical Academy of Social Sciences, Acta 17, 2012 www.pass.va/content/dam/scienzesociali/pdf/acta17/acta17-mouzelis.pdf Modernity: Religious Trends Nicos Mouzelis By conceptualising modernity in sociostructural rather than cultural terms, I will try (a) to show how modernity’s sociostructural features are linked to religious developments – particularly in the anglosaxon world; (b) to examine critically the ongoing secularization debate in the social sci- ences. Modernity can be seen as the type of social organization which be- came dominant in the west after the English industrial revolution and the French revolution. It entails three broad structural traits which render mod- ern society unique – unique in the sense that the above characteristics, in their combination, are not to be found in any pre-modern social formation. These characteristics are: – The demise of segmental localism and the mobilisation/inclusion of a whole population into the national centre/nation state; – The overall differentiation of institutional spheres; – The spread of individualization from the elite to the non elite level. 1. Massive inclusion into the national centre: The process of religious rationalization A) Employing Durkheimian terminology, one can argue that pre-modern, traditional communities had a non-differentiated, segmental social organiza- tion. In this respect they were self-sufficient, relatively autonomous vis-à-vis more inclusive social units. In the west, this localist self-containment/autonomy was first undermined by the absolutist model of governance which took its more developed form in Louis XIV’s France.1 Given technological develop- 1 The French monarchy and its administration, as it was finally shaped under Louis XIV, was the prototype of European absolutist rule, a model imitated all over Europe. -

From Human Prehistory to the Early Civilizations Human Life in the Era
Chapter I From Human Prehistory to the Early Civilizations One day in 10,000 B.C.E. a solitary figure walked by the edge of the Pecos River in the American Southwest. He may have been out hunting or traveling between settlements, but he stopped there to gather up some dead grass and driftwood into a pile. He used his sharpened spear to cut a dead twig from an overhanging cottonwood tree and took a long, dried yucca leaf from his leather belt. He knelt down and held the twig upright on the centerline of the leaf. Then, as he had done many times before, he twirled the stick between his hands until the friction between twig and leaf produced a gleam, or glowing ember, which he quickly transferred to the grass and wood he had gathered. He tended the flame until it grew into a fire that provided not only some warmth, but a means of cooking a meal. When he subsequently rejoined others of his kind, he may have talked about his journey and how he lost his yucca leaf fire-started at that campsite by the river. Of course we have no evidence of his conversation, just the yucca leaf he left behind, found by an archaeologist more than 9000 years later. Our Neolithic (New Stone Age) traveler sends us a number of messages about early world history. Most obviously, he was a tool user who not only picked up natural objects but deliberately crafted them to hunt for and prepare his food. As such, he differed from all other animals (a few other kinds of animals are tool users, but none make their tools).