Ralph William Pridmore
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LED Color Mixing: Basics and Background
CLD-AP38 REV 0A REV CLD-AP38 TECHNICAL ARTICLE LED Color Mixing: Basics and Background INTRODUCTION TABLE OF CONTENTS This technical article explains a few approaches to The Need for Color Consistency ............................ 2 creating color-consistent, LED-based illumination The Basic Approaches ..................................... 4 products and guides readers in how to work effectively Led Binning ....................................................... 4 with Cree products to achieve this goal. Chromaticity Bins ........................................... 4 Flux Bins ....................................................... 6 LEDs, as with all semiconductor devices, have Using Colorimetry and Binning Information in material and process variation which yields product Illumination Specification ..................................... 7 with corresponding variation in performance. LEDs Three Approaches ............................................... 9 are binned and packaged to balance the nature of Buy Single (or Few) Chromaticity Bins ............... 9 manufacturing process with the needs of the lighting Use Cree EasyWhite Parts .............................. 10 industry. Lighting-class LED products are driven Do Color Mixing in the LED System ................. 10 by the needs of the solid-state-lighting industry, Cree’s Color Mixing Tool, the Binonator ............ 14 application requirements and industry standards, Conclusions ..................................................... 18 including color consistency, as well as color -

LED Color Mixing: Basics and Background
CLD-AP38 REV 1D TECHNICAL ARTICLE LED Color Mixing: Basics and Background TABLE OF CONTENTS INTRODUCTION Introduction ...................................................................................1 This application note explains aspects of the theory and practice The Need for Color Consistency in LED Illumination ..................2 of creating color-consistent, LED-based illumination products LED Binning ...................................................................................3 and shows how to use Cree XLamp® LEDs to achieve this end. Colorimetry and Binning Basics ...................................................3 LEDs, as with all manufactured products, have material and Color-Space Basics .................................................................4 process variations that yield products with corresponding Idealized Illumination Colors – the Black Body Curve ..........7 variation in performance. LEDs are binned and packaged to MacAdam Ellipses: The Variability of Perception, Expressed balance the nature of the manufacturing process with the in Color Space .........................................................................9 needs of the lighting industry. Lighting-class LEDs are driven Partitioning the Color Space – Binning ................................11 by application requirements and industry standards, including Chromaticity Bins ..................................................................13 color consistency and color and lumen maintenance. Just as Flux Bins .................................................................................14 -

Inter-Society Color Council News
Inter-Society Color Council News Number 362 July/August 1996 N E FROM THE PRESIDENT'S DESK '/r ~ould like to ~ egi~ by thanking Hugh Fairman and Danny ,D R1ch for the f1ne JOb they did in organizing this year's Annual Meeting at the Doubletree Guest Suites in Orlando, From The President's Desk ....................................... 1 FL. Both the ISCC meeting and the Symposium provided lots of interesting presentations and time to get together with Report-ISCC 65th Annual fv\eeting ........................... 2 colleagues for discussion and fun. Most of the rest of this newsletter is devoted to reports on various activities rel ated to Macbeth Award ..................................................... 10 the Annual Meeting. I would also like to take this oppotunity to thank the New ISCC Officers .............................................. ... 13 outgoing Board members and to welcome the new Directors. Completing their terms on the Board of Directors are Gary Obituary ................................................................ 14 Beebe, Joseph Campbell, and Ro bert Marcus. Speaking for all the officers, I want to say that we appreciate all that they Report-ISCC Committee 50 ............. ....................... 15 have done. Joe organized the instrument displays at the Annual Meetings for several years, Gary is chair of the 1997 New Members ........... .................... ........................ 15 Annual Meeting, and Bob continues as Publicity Chairman and Chair of the Nickerson Service Award Committee. 1a m Birthday Celebration .............................................. 16 happy to be able to report that they will continue to be active in the ISCC community. In This Issue of CR&A ............................................. 17 This spring's elections bring us three new Directors. Serving until 1999, are Helen Epps from the University of Letter of Appreciation ....... -
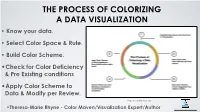
Know Your Data. • Select Color Space & Rule. • Build Color
THE PROCESS OF COLORIZING A DATA VISUALIZATION • Know your data. • Select Color Space & Rule. • Build Color Scheme. •Check for Color Deficiency & Pre Existing conditions •Apply Color Scheme to Data & Modify per Review. Image: Theresa-Marie Rhyne 2021 •Theresa-Marie Rhyne - Color Maven/Visualization1 Expert/Author [email protected] (1) KNOW OR IDENTIFY THE NATURE OF YOUR DATA • Nominal - name/no order-e.g. eye color. • Ordinal - rank - low, medium, high - no info on degree of difference. • Interval - differentiated by degree of difference - numerical values have positive, negative or zero. • Ratio - data distinguished by the degree of difference - Age, Height, Duration • Discrete - only whole numbers & some kind of count • Continuous - data take any value - computational Chart prepared by Georges Hattab, see: Ten simple rules to colorize biological data visualization by Hattab, Rhyne & Heider, model https://journals.plos.org/ploscompbiol/article?id=10.1371/ journal.pcbi.1008259 Binary/Dichotomous - 2 possible values- Yes or No • 2 [email protected] (1) KNOW OR IDENTIFY THE NATURE OF YOUR DATA For the example I will work through in this talk: I want to build a set of bar charts using the 5 point Likert scale of Strongly Agree, Agree, Neutral, Disagree and Strongly Disagree. Magenta is my Key Color. • Ordinal - categorical attributes of a Image: Theresa-Marie Rhyne 2021 variable differentiated by order (rank, scale, or position) Getting it Right in Black & White. • Key Color: Magenta (#FC56FF in Hex Code) 3 [email protected] (2A) SELECT COLOR SPACE & RULE: 3 CLASSIC MODELS RGB adds with lights. CMYK subtracts for printing. RYB subtracts to mix paints. -

Inter-Society Color Council News
Inter-Society Color Council News Number 298 November-December 1985 HELP WANTED Wintertur; Max Saltzman: Elizabeth FitzHugh, Freer Ga llery: I he pll'>l'nt editor of the ISCT ~ews is resigning Robert Feller, Mellon; W. A. Thornton, Prime-Color: and Jolm allll we arc actively looking for a new editor. If Asm us, USC . Ample time will be ava ilable to visit the restoration of anyone is in terested in ben11ning a more active Colonial Will iamsburg which is within short walking distance partk!pant in the ISC'C and wou ld like to volu n of the lodge. An additional attraction this year is the newly teer to help please w ntact Joyce Davenport. See opened DeWitt Wallace Decorator Arts Gallery. This conrains many British and American antiques of the 17th and I 8th cen the last page of this issue for he r address and phone turies, never before exhibited because they did not relate di nunther:Washington. D.C. area lSCT members rectly to Williamsburg. please note. Registration forms may be obtained from 1 orman W. Burningham, 357 True Hickory Dr .. Rochester, .Y. 14615. UPCOMI NG 1986 ANNUAL MEETING CONFERENCE ON ART RESTORATION The 1986 Annual ISCC meeting will be held at Ryerson Poly The Inter-Society Color Council will hold a conference en te chn ical Institute in Toronto, Ontario, Ca nada from June titled, "Colorsof History: Identification, Re-Creation, Preser 15 th through 18th. It will be a joint meeting with the Canadian vat ion," at the Lodge, Colonial Williamsburg , February 9 to Society for Color. -

White Paper an Introduction to Color for Medical Imaging
White Paper An Introduction to Color for Medical Imaging Geert Carrein Johan Rostang Bastian Piepers Albert Xthona What’s inside? • A primer on color • Human vision and color perception • From DICOM gray JND to color JND • Challenges for medical color displays • The relevance of color for medical imaging - Absolute color - Prior segmentation to a certain color - Consistency in calculated colors - Image fusion of multiple modalities page 2 Abstract Over the past several years, we have seen a shift in medical displays from monochrome to color image presentation. During the early days of this transition, color was primarily used to offer the radiologist a much richer user interface or support the use of color annotations. Since then the use of color in medical imaging has dramatically increased. Many of today’s emerging medical applications utilize color to denote a specific meaning, such as in ultrasound, PET-CT fusion and microscopy. Based on currently available imaging technologies, it is clear that additional display requirements are necessary to ensure the accurate, and consistent, display of specific colors over time and space. This paper will briefly introduce the theory of color and describe some of the challenges and considerations for medical color imaging with respect to diagnostic displays. 1 A primer on color 4 2 Human vision and color perception 6 2.1 Color perception by the human eye 7 2.2 Introduction to colorimetry: the cie 1931 xyz coordinate system 8 3 From Dicom gray JND to color JND 10 3.1 Just noticeable difference (jnd) 11 3.2 Color jnd 13 4 Challenges for medical color displays 14 4.1 Stability 15 4.2 Accuracy 15 5 Medical relevance of color 18 5.1 The medical relevance of a perceptually uniform color calibrated display 9 5.2 Clearbase or bluebase 20 6 Conclusion 21 7 References 23 page 3 A primer on color page 4 1. -

The Construction of Colorimetry by Committee. Science in Context 9(4):Pp
Johnston, S. F. (1996) The construction of colorimetry by committee. Science in context 9(4):pp. 387-420. http://eprints.gla.ac.uk/archive/2904/ Glasgow ePrints Service http://eprints.gla.ac.uk Science in Context 9 (1996), 387-420 The Construction of Colorimetry by Committee1 Sean F. Johnston The Argument This paper explores the confrontation of physical and contextual factors involved in the emergence of the subject of color measurement, which stabilized in essentially its present form during the interwar period. The contentions surrounding the specialty had both a national and a disciplinary dimension. German dominance was curtailed by American and British contributions after World War I. Particularly in America, communities of physicists and psychologists had different commitments to divergent views of nature and human perception. They therefore had to negotiate a compromise between their desire for a quantitative system of description and the perceived complexity and human-centeredness of color judgement. These debates were played out not in the laboratory but rather in institutionalized encounters on standards committees. Groups such as this constitute a relatively unexplored historiographic and social site of investigation. The heterogeneity of such committees, and their products, highlight the problems of identifying and following such ephemeral historical 'actors'. Introduction Today, the colors produced by computer screens, printing inks and other products are described by an international convention known as the CIE color system. In this system, a color is described by three numbers, relating indirectly to three ‘primary’ color components of blue, green and red. Color itself is classified as a ‘psychophysical’ phenomenon, a product of physical stimuli, physiology and mental response. -
Measuring Chromaticity and Color Temperature of White Leds by Miriam Mowat, Applications Scientist
Emissive Color Measurement: Measuring Chromaticity and Color Temperature of White LEDs by Miriam Mowat, Applications Scientist Accurate measurement of emissive color is an important application of spectroscopy. LED manufacturers use color measurement for binning their products (i.e., sorting by performance and other criteria) to ensure product consistency and quality. Display manufacturers use emissive color measurements to calibrate the color of display screens. Emissive measurements of LEDs are also useful in horticulture (1), where LEDs are part of plant research and greenhouse lighting; and in biomedical applications, such as the work conducted by NASA on the use of LEDs for stimulation of cell growth (2). In this application note we will compare emissive color measurements of a NIST (National Institute of Standards and Technology) calibrated white LED from two Ocean Optics spectrometers: the compact STS microspectrometer and the new Flame spectrometer. Flame is the next generation of miniature spectrometers from Ocean Optics, delivering high thermal stability and low unit to unit variation while offering interchangeable slits and simple device connectors. We compared the STS and Flame spectrometers on three primary measurement criteria: speed, accuracy and repeatability. Color Measurement Defining the color of something is tricky at best. The way human eyes perceive color is not trivial to replicate. In the 20th century, several methods for defining color were developed. The coordinate system CIE XYZ 1931 is often used, with X and Z referring to chromaticity (hue) and Y referring to luminance (intensity). Figure 1 shows the CIE 1931 X and Y color space. An alternative coordinate system, CIE L*a*b*, is also commonly used, with L* referring to lightness (intensity), a* to red/green chromaticity and b* to yellow/blue chromaticity. -
Pointer's Gamut, Macadam Limits, and Wide-Gamut Displays
© 2016 Phil Service ([email protected]) Last revised: 21 June 2016 Pointer’s Gamut, MacAdam Limits, and Wide-Gamut Displays Phil Service Flagstaff, Arizona, USA 23 May 2016 Summary Three “wide-gamut” RGB color spaces — AdobeRGB, DCI-P3, and Rec. 2020 — are compared to the gamut of real surface colors (Pointer’s gamut) and the optimal color solid (MacAdam limits). Only Rec. 2020 covers Pointer’s gamut completely, or very nearly so, at all luminance levels examined. Rec. 2020 also approaches or exceeds the MacAdam limits in all color regions at high luminance (L* ≥ 80). Better coverage of the MacAdam limits at lower luminance levels will most likely require multi-primary displays. Key words: Pointer’s gamut, MacAdam limits, surface colors, optimal colors, AdobeRGB, DCI-P3, Rec. 2020, wide-gamut displays, Rec. 2020-to-XYZ conversion matrix, DCI-P3-to-XYZ conversion matrix. 1. Introduction 1.1 Optimal Colors and MacAdam Limits Almost everything we can see, we see by reflected light. An object has color because it selectively absorbs some wavelengths of light, and reflects others. In 1917, the German chemist Wilhelm Ostwald proposed that the most saturated reflective colors possible have a very special spectrum: at any given wavelength, they are either complete absorbers or complete reflectors. Furthermore, across the visible spectrum, there can be at most only two transitions between the Type 1 Type 2 100 100 Reflectance, % Reflectance, % 0 0 400 500 600 700 800 400 500 600 700 800 Wavelength, nm Wavelength, nm Fig. 1. Examples of the two spectral reflectance patterns of optimal colors. -

The Construction of Colorimetry by Committee1
Science in Context 9 (1996), 387-420 The Construction of Colorimetry by Committee1 Sean F. Johnston The Argument This paper explores the confrontation of physical and contextual factors involved in the emergence of the subject of color measurement, which stabilized in essentially its present form during the interwar period. The contentions surrounding the specialty had both a national and a disciplinary dimension. German dominance was curtailed by American and British contributions after World War I. Particularly in America, communities of physicists and psychologists had different commitments to divergent views of nature and human perception. They therefore had to negotiate a compromise between their desire for a quantitative system of description and the perceived complexity and human-centeredness of color judgement. These debates were played out not in the laboratory but rather in institutionalized encounters on standards committees. Groups such as this constitute a relatively unexplored historiographic and social site of investigation. The heterogeneity of such committees, and their products, highlight the problems of identifying and following such ephemeral historical 'actors'. Introduction Today, the colors produced by computer screens, printing inks and other products are described by an international convention known as the CIE color system. In this system, a color is described by three numbers, relating indirectly to three ‘primary’ color components of blue, green and red. Color itself is classified as a ‘psychophysical’ phenomenon, a product of physical stimuli, physiology and mental response. This seemingly straightforward scheme of expressing color characteristics was adopted and elaborated before the Second World War, and widely applied after it. The modern consensus regarding this standard, however, masks deep national and disciplinary divisions that surrounded its introduction, and that questioned its implicit foundations and adequacy. -
371 January/February 1998 I· N 21ST CENTURY TECH NO LOGY and CREATIVITY 1998 Williamsburg Conference
Inter-Society Color Council News Number 371 January/February 1998 I· N 21ST CENTURY TECH NO LOGY AND CREATIVITY 1998 Williamsburg Conference ................................ 1 Color Cleaver ........... ........................ ..................... ... 1 1998 WILLIAMSBURG 1998 ISCC/OSA Annual Meeting .............................. 2 CONFERENCE Interest Group I Call For Papers ................................ 3 In This Issue of CR&A ............................................... 3 (FEBRUARY 22-24) GIA 1999 Symposium Cal l for Papers ...................... 4 Fifth Color Imaging Conference Bigger than ever ..... 5 1'i'lr'he Inter -Society Color Counci I wi II be presenting an USNC/CIE ........ ........................................................ 6 ~o utstanding roster of professionals within design, the fine arts and industry who will address issues related AATCC ................. .. ............... ................................... 7 to color as we approach the new millennium. These are CORM 98 ····························································· ··· 7 individuals who have made major contributions to their FSCT ......................................... ............................... 8 disciplines on a national and international level. The two and one-half day program will include opportunities for RIT Munsell Color Lab 1998 Industrial Short Course 9 speakers and attendees to interact within open scheduled News From Members ............................................. 10 discussions and social events. Time has been allotted -

Inter-Society Color Council News
Inter-Society Color Council News SUPPORT YOUR JOURNAL! Number 283 MARCH-APRIL 1983 For many years those of us who were attempting to deal with color as a science and as a technology moped about and bit terly complained about the multiple sources we had to con etry, optimal colours, dominant wavelength and excitation sult in the li terature in order to obtain a reasonable knowledge purity, uniform chromaticity diagrams and uniform colour of current developments. In 197 5 this all changed. solids for renecting objects, perceptible differences in chroma The Inter-Society Color Council and John Wiley and Sons ticity and in tristimulus val ues leading to the well-known concluded in an agreement whereby Color Research and Appli MacAdam ellipses and ellipsoids, chro matic adaptation, colour cation became THE journal endorsed by the Inter-Society computations including some of the fi rst use of computers, Color Council. Tllis was an absolutely marvelous event, and loci of constant hue and brightness, an d colorimetric funda one that is now appreciated worldwide . Each new member of mentals of colour reproduction especially in colour photog ISCC is urged to subscribe to the journal in o rder to be in raphy, are among the contributions no ted here for recognition touch with his (or her) peers. The variety of articles in this by the Association Internationale de la Couleur. His profes outstanding publication is easily appreciated with every issue sional career has extended over fifty years: he was a physicist by simply scanning the Table of Contents. in the Kodak Research Laboratories at Rochester from 1936 However, not all of ISCC members have, in fac t, subscribed untill975, finishing as a Senior Research Associate; he was to the journal.