BMC Evolutionary Biology Biomed Central
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylogenetic Reanalysis of Strauch's Osteological Data Set for The
TheCondor97:174-196 0 The Cooper Ornithological Society 1995 PHYLOGENETIC REANALYSIS OF STRAUCH’S OSTEOLOGICAL DATA SET FOR THE CHARADRIIFORMES PHILIP c. CHU Department of Biology and Museum of Zoology The University of Michigan, Ann Arbor, MI 48109 Abstract. Strauch’s (1978) compatibility analysisof relationshipsamong the shorebirds (Charadriifonnes) was the first study to examine the full range of charadriifonn taxa in a reproducibleway. SubsequentlyMickevich and Parenti (1980) leveled seriouscharges against Strauch’s characters,method of phylogenetic inference, and results. To account for these charges,Strauch ’s characterswere re-examined and recoded, and parsimony analyseswere performed on the revised matrix. A parsimony analysison 74 taxa from the revised matrix yielded 855 shortesttrees, each length = 286 and consistencyindex = 0.385. In each shortest tree there were two major lineages,a lineageof sandpiper-likebirds and a lineageof plover- like birds; the two formed a monophyletic group, with the auks (Alcidae) being that group’s sister taxon. The shortest trees were then compared with other estimates of shorebird re- lationships, comparison suggestingthat the chargesagainst Strauch’s results may have re- sulted from the Mickevich and Parenti decisions to exclude much of Strauch’s character evidence. Key words: Charadrilformes; phylogeny; compatibility analysis: parsimony analysis; tax- onomic congruence. INTRODUCTION Strauch scored 227 charadriiform taxa for 70 The investigation of evolutionary relationships characters. Sixty-three of the characters were among shorebirds (Aves: Charadriiformes) has a taken from either the skull or postcranial skel- long history (reviewed in Sibley and Ahlquist eton; the remaining seven involved the respec- 1990). Almost all studies used morphology to tive origins of three neck muscles, as published make inferences about shared ancestry; infer- in Burton (1971, 1972, 1974) and Zusi (1962). -

Complete Species Table in Species Number Order
Page 1 of 19 Complete Species Table in Species Number order Go to species 100 .0, 200 .0, 300 .0, 400 .0, 500 .0, 600 .0, 700 .0, 800 .0, 900 .0 SPECIES COMMON NAME ALPHA CODE BAND SIZE 001 .0 Western Grebe WEGR 7A 7B 001 .1 Clark's Grebe CLGR 7A 7B 002 .0 Red-necked Grebe RNGR 7A 003 .0 Horned Grebe HOGR 6 5 004 .0 Eared Grebe EAGR 5 005 .0 Least Grebe LEGR 4 006 .0 Pied-billed Grebe PBGR 5 6 007 .0 Common Loon COLO 8 008 .0 Yellow-billed Loon YBLO 9 009 .0 Arctic Loon ARLO 7B 010 .0 Pacific Loon PALO 7B 011 .0 Red-throated Loon RTLO 7B 012 .0 Tufted Puffin TUPU 6 5 013 .0 Atlantic Puffin ATPU 5 014 .0 Horned Puffin HOPU 5 015 .0 Rhinoceros Auklet RHAU 5 6 016 .0 Cassin's Auklet CAAU 3B-3A 017 .0 Parakeet Auklet PAAU 4 018 .0 Crested Auklet CRAU 4 019 .0 Whiskered Auklet WHAU 3 020 .0 Least Auklet LEAU 2 3 021 .0 Ancient Murrelet ANMU 3B 3 023 .0 Marbled Murrelet MAMU 3B 3 023 .1 Long-billed Murrelet LBMU 3B 3 024 .0 Kittlitz's Murrelet KIMU 3B 025 .0 Xantus's Murrelet XAMU 2 026 .0 Craveri's Murrelet CRMU 2 027 .0 Black Guillemot BLGU 4 029 .0 Pigeon Guillemot PIGU 4A 030 .0 Common Murre COMU 6M 031 .0 Thick-billed Murre TBMU 6M 5R 032 .0 Razorbill RAZO 5R 034 .0 Dovekie DOVE 3 035 .0 Great. -
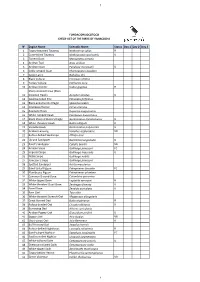
N° English Name Scientific Name Status Day 1
1 FUNDACIÓN JOCOTOCO CHECK-LIST OF THE BIRDS OF YANACOCHA N° English Name Scientific Name Status Day 1 Day 2 Day 3 1 Tawny-breasted Tinamou Nothocercus julius R 2 Curve-billed Tinamou Nothoprocta curvirostris U 3 Torrent Duck Merganetta armata 4 Andean Teal Anas andium 5 Andean Guan Penelope montagnii U 6 Sickle-winged Guan Chamaepetes goudotii 7 Cattle Egret Bubulcus ibis 8 Black Vulture Coragyps atratus 9 Turkey Vulture Cathartes aura 10 Andean Condor Vultur gryphus R Sharp-shinned Hawk (Plain- 11 breasted Hawk) Accipiter striatus U 12 Swallow-tailed Kite Elanoides forficatus 13 Black-and-chestnut Eagle Spizaetus isidori 14 Cinereous Harrier Circus cinereus 15 Roadside Hawk Rupornis magnirostris 16 White-rumped Hawk Parabuteo leucorrhous 17 Black-chested Buzzard-Eagle Geranoaetus melanoleucus U 18 White-throated Hawk Buteo albigula R 19 Variable Hawk Geranoaetus polyosoma U 20 Andean Lapwing Vanellus resplendens VR 21 Rufous-bellied Seedsnipe Attagis gayi 22 Upland Sandpiper Bartramia longicauda R 23 Baird's Sandpiper Calidris bairdii VR 24 Andean Snipe Gallinago jamesoni FC 25 Imperial Snipe Gallinago imperialis U 26 Noble Snipe Gallinago nobilis 27 Jameson's Snipe Gallinago jamesoni 28 Spotted Sandpiper Actitis macularius 29 Band-tailed Pigeon Patagoienas fasciata FC 30 Plumbeous Pigeon Patagioenas plumbea 31 Common Ground-Dove Columbina passerina 32 White-tipped Dove Leptotila verreauxi R 33 White-throated Quail-Dove Zentrygon frenata U 34 Eared Dove Zenaida auriculata U 35 Barn Owl Tyto alba 36 White-throated Screech-Owl Megascops -

BIRDS OBSERVED at SEA DURING the VOYAGE of HMS BLONDE to HAWAII [1824-1826) by Storrs L
ROVAL NAVAL BIRDWATCHING SOCIETY (1995) • Sea Swallow 44. BIRDS OBSERVED AT SEA DURING THE VOYAGE OF HMS BLONDE TO HAWAII [1824-1826) By Storrs L. Olson. It seems appropriate to put on record here some reports of birds observed at sea aboard a vessel of the Royal Navy that have remained unpublished for almost a century and three-quarters. In 1824 the 46-gun frigate Blonde departed England on a diplomatic mission to the Hawaiian Islands, under the command of George Anson, Lord Byron, who had succeeded to that title only a few months previously, upon the death of his cousin, the renowned poet. The Blonde crossed the Atlantic to Brazil by way of Madeira, sailed round Cape Horn, stopping in Chile, Peru, and the Galapagos, before arriving in Hawaii. She returned in 1826 via Maiden Island, Mauke [in the Cook group), Chile, and St. Helena. Aboard was a youthful and largely untrained naturalist, Andrew Bloxam, who prepared specimens and kept natural history notes during the voyage. Apart from a few observations incidentally included in a volume that appeared under Byron's name [Byron 1827] that was cobbled together by a compiler from several diaries, virtually nothing was published on the natural history of the voyage until I began my studies of Bloxam's manuscript natural history notes, which are housed at the British Museum [Natural History) [Olson 1986, in press a,b). These notes contain several references to landbirds observed at sea, as well as seabirds. There are several transcriptions of Bloxam's notes. The passages that follow are from a fair copy, with additional or variant information from rougher notes inserted in brackets [ ]. -

Recommended English Names
86 BIRDS OF THE WORLD: RECOMMENDED ENGLISH NAMES Gill, F.B. & Wright, M. 2006. Princeton, NJ, and London, UK: Princeton University Press. 259 pp. with CD containing Microsoft Excel spreadsheets of species lists. Soft cover. ISBN 0691128278. US$20. Birds are among the few taxa for which non-scientific vernacular In addition to competing English names, the IOC committee had names are extensively used in scientific communication as well to deal with ongoing taxonomic shuffling too. It isn’t clear how as in the burgeoning birding community. Consequently, there is a they reached consensus here, but some recent splits are recognized need to have some consistency in the names used around the world. (e.g. Nazca Booby, Vega Gull, the various sub-Antarctic shags), and The International Ornithological Congress (IOC) sought to reach others are not (e.g. the suggested splits of Wandering, Yellow-nosed consensus in vernacular names for commonly used languages. and other albatrosses). As the editors reiterate, this compilation is a Standardized names have been published for French (Devillers work in progress, and future versions will undoubtedly address the and Ouellet 1993) and Spanish (Bernis 1995). The goal of Birds changing taxonomic landscape. of the World: Recommended English Names is to promote a set of unique English names for the extant bird species of the world. Led Everyone who peruses these lists will be disappointed to see some old originally by the now-late Burt Monroe, and then Frank Gill and favourites voted out and will find fault with some decisions (I think I Minturn Wright, an IOC committee and regional subcommittees hear the rumble of European dissent over their guillemots and divers). -

Avian Nesting and Roosting on Glaciers at High Elevation, Cordillera Vilcanota, Peru
The Wilson Journal of Ornithology 130(4):940–957, 2018 Avian nesting and roosting on glaciers at high elevation, Cordillera Vilcanota, Peru Spencer P. Hardy,1,4* Douglas R. Hardy,2 and Koky Castaneda˜ Gil3 ABSTRACT—Other than penguins, only one bird species—the White-winged Diuca Finch (Idiopsar speculifera)—is known to nest directly on ice. Here we provide new details on this unique behavior, as well as the first description of a White- fronted Ground-Tyrant (Muscisaxicola albifrons) nest, from the Quelccaya Ice Cap, in the Cordillera Vilcanota of Peru. Since 2005, .50 old White-winged Diuca Finch nests have been found. The first 2 active nests were found in April 2014; 9 were found in April 2016, 1 of which was filmed for 10 d during the 2016 nestling period. Video of the nest revealed infrequent feedings (.1 h between visits), slow nestling development (estimated 20–30 d), and feeding via regurgitation. The first and only active White-fronted Ground-Tyrant nest was found in October 2014, beneath the glacier in the same area. Three other unoccupied White-fronted Ground-Tyrant nests and an eggshell have been found since, all on glacier ice. At Quelccaya, we also observed multiple species roosting in crevasses or voids (caves) beneath the glacier, at elevations between 5,200 m and 5,500 m, including both White-winged Diuca Finch and White-fronted Ground-Tyrant, as well as Plumbeous Sierra Finch (Phrygilus unicolor), Rufous-bellied Seedsnipe (Attagis gayi), and Gray-breasted Seedsnipe (Thinocorus orbignyianus). These nesting and roosting behaviors are all likely adaptations to the harsh environment, as the glacier provides a microclimate protected from precipitation, wind, daily mean temperatures below freezing, and strong solar irradiance (including UV-B and UV-A). -

Argentina & Chile
Argentina & Chile Southern Patagonia & Torres del Paine 3rd December to 13th December 2022 (11 days) Magellanic Plover by Rich Lindie Our tour through Argentina’s Southern Patagonia takes us on an amazing adventure through the southern portion of this incredible continent in search of the unique Magellanic Plover at Laguna Nímez, the rare White-bellied Seedsnipe on the wide-open grassy plains of northern Tierra del Fuego, and the charismatic Magellanic Woodpecker. Our journey begins in Los Glaciares National Park, famous in birding circles for its population of the impressive Andean Condors, uncommon Bronze-winged Duck, Chilean Flicker and the strange Rufous-tailed Plantcutter. Crossing the border into Chile, we spend a few days at possibly the most scenically impressive site on a tour of grand vistas - Torres del Paine. Aside from being a staggeringly spectacular stretch of mountains - quite possibly the most attractive scenery of all the Andes - Torres del Paine National Park also happens to be one of the best places in the world to see the mighty Puma. Next, we travel to the Punta Arenas, exploring the most southern continental locations for RBL Argentina - Southern Patagonia Itinerary 2 their plethora of rare and endemic species, both by road and ferry. As the tour draws to a close, we will be searching for birds in the dramatic and fabled landscapes of Tierra del Fuego. Here a visit to Tierra del Fuego National Park could produce Austral Pygmy Owl, White-throated Treerunner and the fantastic Magellanic Woodpecker. In the Beagle Channel, we hope for Magellanic Diving Petrel, Fuegian Steamer Duck and White-throated Caracara, while in the Rio Grande area we search for the rare White-bellied Seedsnipe. -

Bolivia: Endemic Macaws & More!
BOLIVIA: ENDEMIC MACAWS & MORE! PART II: FOOTHILLS, CLOUDFORESTS & THE ALTIPLANO SEPTEMBER 28–OCTOBER 8, 2018 Male Versicolored Barbet – Photo Andrew Whittaker LEADERS: ANDREW WHITTAKER & JULIAN VIDOZ LIST COMPILED BY: ANDREW WHITTAKER VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM Bolivia continued to exceed expectations on Part 2 of our tour! Steadily climbing up into the mighty ceiling of South America that is the Andes, we enjoyed exploring many more new, different, and exciting unspoiled bird-rich habitats, including magical Yungas cloudforest stretching as far as the eye could see; dry and humid Puna; towering snow-capped Andean peaks; vast stretches of Altiplano with its magical brackish lakes filled with immense numbers of glimmering flamingoes, and one of my favorite spots, the magnificent and famous Lake Titicaca (with its own flightless grebe). An overdose of stunning Andean scenery combined with marvelous shows of flowering plants enhanced our explorations of a never-ending array of different and exciting microhabitats for so many special and interesting Andean birds. We were rewarded with a fabulous trip record total of 341 bird species! Combining our two exciting Bolivia tours (Parts 1 and 2) gave us an all-time VENT record, an incredible grand total of 656 different bird species and 15 mammals! A wondrous mirage of glimmering pink hues of all three species of flamingos on the picturesque Bolivian Altiplano – Photo Andrew Whittaker Stunning Andes of Bolivia near Soroto on a clear day of our 2016 trip – Photo Andrew Whittaker Victor Emanuel Nature Tours 2 Bolivia Part 2, 2018 We began this second part of our Bolivian bird bonanza in the bustling city of Cochabamba, spending a fantastic afternoon birding the city’s rich lakeside in lovely late afternoon sun. -

Transport of Water by Adult Sandgrouse to Their Young Tom J
THE CONDOR VOLUME69 JULY-AUGUST,1967 NUMBER4 TRANSPORT OF WATER BY ADULT SANDGROUSE TO THEIR YOUNG TOM J. CADE and GORDONL. MACLEAN In 1896 the English aviculturist Meade-Waldo published an astonishing and seemingly incredible account of how the males of sandgrouse that he successfully bred in captivity carried water to their young in their breast feathers. To quote from his original report: As soon as the young were out of the nest (when twelve hours old) a very curious habit developed itself in the male. He would rub his breast violently up and down on the ground, a motion quite distinct from dusting, and when all awry he would get into his drinking water and saturate the feathers of the under parts. When soaked he would go through the motions of flying away, nodding his head, etc. Then, remembering his family were close by, would run up to the hen, make a demonstration, when the young would run out, get under him, and suckthe water from his breast. This is no doubt the way that water is conveyed to the young when far out on waterless plains. The young . are very independent, eating hard seed and weeds from the first, and roosting independently of their parents at ten days old (Meade-Waldo, 1896). See also Meade- Waldo (1921). Despite the fact that .Meade-Waldo (1897 ; 1921) observed 61 broods from three different species of sandgrouse hatched in his aviaries between 189.5 and l915, and soon received confirmation from another breeder for two species (St. Quintin, 1905), and despite the fact that field naturalists and native hunters have frequently observed wild male sandgrouse wetting their breast feathers at water holes in the way described (Meade-Waldo, 1906; Buxton, 1923; Heim de Balsac, 1936; Hoesch, 1955), the idea that the young do receive water in this exceptional way has met with a great deal of scepticism (Archer and Godman, 1937; Meinertzhagen, 1954, 1964; Hiie and Etchkcopar, 1957; Schmidt-Nielsen, 1964). -

Wilson's Snipe
Wilson’s Snipe (Gallinago delicate) Torrey Wenger Monroe Co., MI. 4/10/2007 © Allen Chartier (Click to view a comparison of Atlas I to II) Wilson’s Snipe is a victim of the lumpers and dwelling sandpiper was reported in 23% of Michigan’s townships; in MBBA II, they were splitters. In 1945, Wilson’s Snipe was reported in only 14.5%, a drop of nearly 40%. combined with other snipe species worldwide as The most significant decrease occurred in the the Common Snipe, Gallinago gallinago (AOU SLP: Wilson’s Snipe were found in almost 60% 1945). In 2002, based on behavioral and fewer townships and were not found in 14 structural differences, it was split off again counties where they were previously (Banks et al. 2002). While this frustrates casual documented. birders and competitive listers alike, the bird itself certainly does not care. This on-again, Some of this decline may be caused by observer off-again status is not the origin of the infamous bias. First, snipe are most detectable during “snipe hunt”, where children are sent into the their early mating season, as males make bushes searching for a non-existent bird. “winnowing” flights above their territories – if observers delayed the start of their fieldwork, All snipe feed by using their long bills to probe they missed this species. Second, the snipe is a for small insects and larvae in mud and moist habitat specialist, preferring wetlands with low soils. A snipe can open just the tip of its bill, herbaceous vegetation, sparse shrubs, and enabling it to capture and swallow small prey scattered trees (Mueller 1999) – if observers items without removing its bill from the soil. -

Native Grasslands and the Plains- Wanderer
Birds Australia Conservation Statement No. 1 NATIVE GRASSLANDS AND THE PLAINS- WANDERER by David Baker-Gabb SUMMARY: Lowland native grasslands are among the most depleted ecosystems in south-eastern Australia, and contain a dispro- portionately large number of threatened plant species. Threatened grassland fauna such as the Plains-wanderer have suffered a similar decline. Plains-wanderers are permanent residents in their favoured patches of sparse native grassland. However, they cannot survive where these grasslands are converted to crops or dense introduced pasture, or are overgrazed by stock. In the five years since the publication of the first RAOU Plains-wanderer Conser- vation Statement in 1993, considerable effort has been expended in surveying and studying the temperate native grasslands of South Australia, Victoria and New South Wales. This work has revealed that the status of the Plains-wanderer is worse than previously thought. A very significant new threat has emerged from an expanding rice industry in the Plains-wanderer’s stronghold, the Riverina of New South Wales. The situation for Plains- wanderers in Queensland remains unclear, but there is no reason to suspect that it is improving given the expansion of the cotton industry and conversion of native grasslands to introduced pasture there. On the positive side, recent studies have provided refined information on managing native grasslands to maintain their biodiversity, and they provide some clear targets for urgent conservation action. High quality native grassland with sparse open structure, NSW Riverina – typically favoured Plains- wanderer habitat. Photo by Marianne Porteners/ Royal Botanic Gardens Sydney Female Plains-wanderer. Photo by Tom Wheller Supplement to Wingspan, vol. -

Snowy Sheathbills and What You Really Need to Know
Snowy Sheathbills and what you really need to know Reprinted from British Antarctic Survey Bulletin, No. 2, December 1963, p. 53-71 THE SHEATHBILL, Chionis alba (Gmelin), AT SIGNY ISLAND, SOUTH ORKNEY ISLANDS By N. V. Jones ABSTRACT. A study of the sheathbill, Chionis alba (Gmelin), was carried out at Signy Island, South Orkney Islands, between April 1961 and April 1962. The general characteristics of the species and the annual population cycle have been investigated. Breeding birds return to the island early in October, form pairs early in November, and establish territories later in November. There is a high level of fidelity to a previous year's mate and considerable nest-site tenacity. Nests are concentrated around penguin (Pygoscelis adetiae and P. antarctica) colonies, the closer association being with the latter species. Both sexes participate in nest construction, incubation and the care of the chick. Egg-laying begins in early December, but full incubation starts only when clutches, which number from one to four, are complete. The normal incubation period is 30 days. Chicks are fed largely on "krill", obtained by disturbing penguins which are feeding their own young, and the sheathbill's life cycle is suitably coincident with that of the penguins. Chicks fledge when 50 to 60 days old and at this stage they become shore feeders. Chicks and adults disperse from the island in about mid-May, and there is evidence of a rather loose northward migration in winter. No definite predators are known. A number of parasites have been recorded. THIS paper describes work done on the sheathbill at Signy Island (lat.