Partnerre Trigger Pharmaceuticals (At 9.3.20) Code Brand Name Generic Name Code Brand Name Generic Name J0800 Acthar®
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Oregon Medicaid Pharmaceutical Services Prior Authorization Criteria
Oregon Medicaid Pharmaceutical Services Prior Authorization Criteria HEALTH SYSTEMS DIVISION Prior authorization (PA) criteria for fee-for-service prescriptions for Oregon Health Plan clients March 1, 2021 Contents Contents ................................................................................................................................................................ 2 Introduction........................................................................................................................................................... 7 About this guide ......................................................................................................................................... 7 How to use this guide ................................................................................................................................. 7 Administrative rules and supplemental information .................................................................................. 7 Update information............................................................................................................................................... 8 Effective March 1, 2021 ............................................................................................................................ 8 Substantive updates and new criteria ............................................................................................. 8 Clerical changes ............................................................................................................................ -

Medicines Not Reimbursed Through National Prices and Directly Commissioned by Nhs England V14 Changes to Version 13
MEDICINES NOT REIMBURSED THROUGH NATIONAL PRICES AND DIRECTLY COMMISSIONED BY NHS ENGLAND V14 CHANGES TO VERSION 13 PUBLISHED APRIL 2019 Changes to v13 Lines removed SUITABLE SPECIALIST FOR CENTRE SUITABLE FOR SHARED ONLY SHARED CARE CARE PRIOR (includng WITH PRIMARY BETWEEN STOPPING MONITORING/ AUDIT APPROVAL outreach CARE (IF DRUG NAME INDICATION COMMISSIONER PBR CATEGORY TA/POLICY STARTING CRITERIA SPECIALIST COMMENT CRITERIA REQUIREMENTS PROFORMA when SUPPORTED AND REQUIRED delivered as BY LOCAL SECONDARY part of a PRESCRIBING CARE VIA provider COMMITTEE) NETWORK network) MODEL PAEDIATRIC INDICATIONS (IN LINE WITH NHS ENGLAND AS PER ADULT TA'S (TA195, TA373, ABATACEPT NHS ENGLAND CYTOKINE MODULATORS NICE NICE NICE √ MEDICINES FOR CHILDREN TA375) POLICY) Now covered by comment 8 PAEDIATRIC INDICATIONS (IN AS PER TA455 OR ADULT TA'S (TA130 LINE WITH NHS ENGLAND (replaced by TA375), TA143 (repalced by ADALIMUMAB NHS ENGLAND CYTOKINE MODULATORS NICE NICE NICE AUDIT √ √ MEDICINES FOR CHILDREN TA383), TA146, TA187, TA199, TA329, POLICY) TA392, TA460) Now covered by comment 8 ALBUTREPENONACOG ALFA HAEMOPHILIA B PRODUCTS ON CMU Blood factor products simplified NHS ENGLAND BLOOD-RELATED PRODUCTS SSC 1652 SSC 1652 SSC 1652 √ FRAMEWORK to blood factor product VIII etc PAEDIATRIC INDICATIONS (IN LINE WITH NHS ENGLAND AS PER IFR AS PER IFR ANAKINRA NHS ENGLAND CYTOKINE MODULATORS NOT ROUTINELY COMMISSIONED AS PER IFR APPROVAL √ MEDICINES FOR CHILDREN APPROVAL APPROVAL POLICY) Now covered by comment 8 AS PER BCSH GUIDELINES ANTIHAEMOPHILIC FACTOR/VON -

A Phase 3, Randomized, Double-Blind Study of Adjuvant Cemiplimab Versus Placebo Post-Surgery and Radiation in Patients With
433 A Phase 3, Randomized, Double-Blind Study of Adjuvant Cemiplimab Versus Placebo Post-Surgery and Radiation in Patients with High-Risk Cutaneous Squamous Cell Carcinoma (CSCC) Danny Rischin,1 Matthew G. Fury,2 Israel Lowy,2 Elizabeth Stankevich,3 Siyu Li,2 Hyunsil Han,2 Sandro V. Porceddu4 1Department of Medical Oncology, Peter MacCallum Cancer Centre, Melbourne, Australia; 2Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA; 3Regeneron Pharmaceuticals, Inc., Basking Ridge, NJ, USA; 4School of Medicine, University of Queensland, Herston, Queensland, Australia; Department of Radiation Oncology, Princess Alexandra Hospital, Woolloongabba, Queensland, Australia. Background Figure 1. C-POST study design Table 2. Key exclusion criteria • Squamous cell carcinoma arising from non-cutaneous sites Summary Cutaneous squamous cell carcinoma (CSCC) Patients with high-risk CSCC often experience relapse with Surgery, with high-risk • Concurrent malignancy other than localized CSCC and/or history of • CSCC is the second most common skin cancer with an estimated features on surgical locoregional recurrence or distant metastases despite initial malignancy other than localized CSCC within 3 years of date of • 1 pathology report incidence of around 1 million cases per year in the US. Worldwide, randomization, except for tumors with negligible risk of metastasis treatment with surgery and post-operative radiation. reports show an annual rise in incidence of 3–7% in most countries.2 350 mg cemiplimab Optional cemiplimab re-treatment after disease recurrence -

CDER Breakthrough Therapy Designation Approvals Data As of December 31, 2020 Total of 190 Approvals
CDER Breakthrough Therapy Designation Approvals Data as of December 31, 2020 Total of 190 Approvals Submission Application Type and Proprietary Approval Use Number Number Name Established Name Applicant Date Treatment of patients with previously BLA 125486 ORIGINAL-1 GAZYVA OBINUTUZUMAB GENENTECH INC 01-Nov-2013 untreated chronic lymphocytic leukemia in combination with chlorambucil Treatment of patients with mantle cell NDA 205552 ORIGINAL-1 IMBRUVICA IBRUTINIB PHARMACYCLICS LLC 13-Nov-2013 lymphoma (MCL) Treatment of chronic hepatitis C NDA 204671 ORIGINAL-1 SOVALDI SOFOSBUVIR GILEAD SCIENCES INC 06-Dec-2013 infection Treatment of cystic fibrosis patients age VERTEX PHARMACEUTICALS NDA 203188 SUPPLEMENT-4 KALYDECO IVACAFTOR 21-Feb-2014 6 years and older who have mutations INC in the CFTR gene Treatment of previously untreated NOVARTIS patients with chronic lymphocytic BLA 125326 SUPPLEMENT-60 ARZERRA OFATUMUMAB PHARMACEUTICALS 17-Apr-2014 leukemia (CLL) for whom fludarabine- CORPORATION based therapy is considered inappropriate Treatment of patients with anaplastic NOVARTIS lymphoma kinase (ALK)-positive NDA 205755 ORIGINAL-1 ZYKADIA CERITINIB 29-Apr-2014 PHARMACEUTICALS CORP metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib Treatment of relapsed chronic lymphocytic leukemia (CLL), in combination with rituximab, in patients NDA 206545 ORIGINAL-1 ZYDELIG IDELALISIB GILEAD SCIENCES INC 23-Jul-2014 for whom rituximab alone would be considered appropriate therapy due to other co-morbidities -

Libtayo® (Cemiplimab-Rwlc)
Libtayo® (cemiplimab-rwlc) (Intravenous) Document Number: IC-0398 Last Review Date: 06/01/2021 Date of Origin: 10/30/2018 Dates Reviewed: 11/2018, 03/2019, 06/2019, 09/2019, 12/2019, 03/2020, 6/2020, 12/2020, 03/2021, 06/2021 I. Length of Authorization Coverage will be provided for six months and may be renewed. II. Dosing Limits A. Quantity Limit (max daily dose) [NDC Unit]: • Libtayo 350 mg/7 mL single-use vial: 1 vial per 21 days B. Max Units (per dose and over time) [HCPCS Unit]: • 350 billable units every 21 days III. Initial Approval Criteria1 Coverage is provided for the following conditions: • Patient is at least 18 years of age; AND Universal Criteria 1 • Patient has not received previous therapy with a programmed death (PD-1/PD-L1)-directed therapy (e.g., avelumab, pembrolizumab, atezolizumab, durvalumab, nivolumab, dostarlimab, etc.), unless otherwise specified; AND • Used as a single-agent therapy; AND • Patient has not received previous therapy with a cytotoxic T-lymphocyte antigen 4 (CTLA-4) targeting agent (e.g., ipilimumab, etc.) within the 4 weeks prior to therapy; AND Cutaneous Squamous Cell Carcinoma (CSCC) † 1-5 • Patient has nodal or distant metastatic disease, locally advanced disease, inoperable or not fully resectable regional disease, or regional recurrence; AND • Patient is not a candidate for curative surgery or curative radiation therapy Basal Cell Carcinoma (BCC) † 1,2,6 • Patient has locally advanced OR nodal, regional, or distant metastatic disease; AND Proprietary & Confidential © 2021 Magellan Health, -

Northwest Medical Benefit Formulary (List of Covered Drugs) Please Read
Northwest Medical Benefit Formulary (List of Covered Drugs) Please Read: This document contains information about the drugs we cover when they are administered to you in a Participating Medical Office. What is the Kaiser Permanente Northwest Medical Benefit Formulary? A formulary is a list of covered drugs chosen by a group of Kaiser Permanente physicians and pharmacists known as the Formulary and Therapeutics Committee. This committee meets regularly to evaluate and select the safest, most effective medications for our members. Kaiser Permanente Formulary The formulary list that begins on the next page provides information about some of the drugs covered by our plan when they are administered to you in a Participating Medical Office. Depending on your medical benefits, you may pay a cost share for the drug itself. The first column of the chart lists the drug’s generic name. The second column lists the brand name. Most administered medications and vaccines are only available as brand name drugs. Contraceptive Drugs and Devices Your provider may prescribe as medically necessary any FDA-approved contraceptive drug or device, including those on this formulary, which you will receive at no cost share. Last Updated 09/01/2021 Generic Name Brand Name Clinic Administered Medications (ADMD) ABATACEPT ORENCIA ABOBOTULINUM TOXIN A DYSPORT ADALIMUMAB HUMIRA ADO-TRASTUZUMAB EMTANSINE KADCYLA AFAMELANOTIDE ACETATE SCENESSE AFLIBERCEPT EYLEA AGALSIDASE BETA FABRAZYME ALDESLEUKIN PROLEUKIN ALEMTUZUMAB LEMTRADA ALGLUCOSIDASE ALFA LUMIZYME ARALAST, GLASSIA, -

CDER Therapeutic Biologic Products List
CDER Therapeutic Biologic Products This list is intended to include all the Center for Drug Evaluation and Research (CDER) user fee billable therapeutic biological products and potencies approved under Section 351 of the Public Health Service Act. The Orange Book includes a section entitled "Drug Products with Approval under Section 505 of the Act Administered by CBER." Included on that list are several products that have been transferred to CDER which would be considered billable also. Program fees are assessed for each potency in which the approved (non-revoked, non-suspended) product is manufactured in final dosage form. When evaluating the specific strength or potency of a drug in final dosage form for purposes of assessing program fees for liquid parenteral biological products, CDER intends to take into consideration both the total amount of drug substance in mass or units of activity in a product and the concentration of drug substance (mass or units of activity per unit volume of product). Biologic products considered to have a different strength or potency in a final dosage form will be given separate entries in the Biologics List and assessed separate program fees. An auto-injector that has the same strength or potency as a prefilled syringe or vial will generally be assessed a separate prescription drug program fee. In certain circumstances, products which have been discontinued from marketing but are still licensed are not assessed program fees. Those products are identified on the CDER Discontinued Biologic Product List section. The potency information contained in this list is based on information in our database. -

Kaiser Permanente Bernard J. Tyson School of Medicine, Inc. Exclusive Provider Organization (EPO) Student Blanket Health Plan Drug Formulary
Kaiser Permanente Bernard J. Tyson School of Medicine, Inc. Exclusive Provider Organization (EPO) Student Blanket Health Plan Drug Formulary Effective September 1, 2021 Health Plan Products: Kaiser Permanente Bernard J. Tyson School of Medicine, EPO Student Blanket Health Plan offered by Kaiser Permanente Insurance Company For the most current list of covered medications or for help understanding your KPIC insurance plan benefits, including cost sharing for drugs under the prescription drug benefit and under the medical benefit: Call 1-800-533-1833, TTY 711, Monday through Friday, 7 a.m. to 9 p.m. ET Visit kaiserpermanente.org to: • Find a participating retail pharmacy by ZIP code. • Look up possible lower-cost medication alternatives. • Compare medication pricing and options. • Find an electronic copy of the formulary here. • Get plan coverage information. For cost sharing information for the outpatient prescription drug benefits in your specific plan, please visit kp.org/kpic-websiteTBD The formulary is subject to change and all previous versions of the formulary are no longer in effect. Kaiser Permanente Last updated: September 1, 2021 Table of Contents Informational Section...........................................................................................................................................3 ANTIHISTAMINE DRUGS - Drugs for Allergy.....................................................................................................9 ANTI-INFECTIVE AGENTS - Drugs for Infections........................................................................................... -

Recent Advances in Oligonucleotide Therapeutics in Oncology
International Journal of Molecular Sciences Review Recent Advances in Oligonucleotide Therapeutics in Oncology Haoyu Xiong 1, Rakesh N. Veedu 2,3 and Sarah D. Diermeier 1,* 1 Department of Biochemistry, University of Otago, Dunedin 9016, New Zealand; [email protected] 2 Centre for Molecular Medicine and Innovative Therapeutics, Murdoch University, Perth 6150, Australia; [email protected] 3 Perron Institute for Neurological and Translational Science, Perth 6009, Australia * Correspondence: [email protected] Abstract: Cancer is one of the leading causes of death worldwide. Conventional therapies, including surgery, radiation, and chemotherapy have achieved increased survival rates for many types of cancer over the past decades. However, cancer recurrence and/or metastasis to distant organs remain major challenges, resulting in a large, unmet clinical need. Oligonucleotide therapeutics, which include antisense oligonucleotides, small interfering RNAs, and aptamers, show promising clinical outcomes for disease indications such as Duchenne muscular dystrophy, familial amyloid neuropathies, and macular degeneration. While no approved oligonucleotide drug currently exists for any type of cancer, results obtained in preclinical studies and clinical trials are encouraging. Here, we provide an overview of recent developments in the field of oligonucleotide therapeutics in oncology, review current clinical trials, and discuss associated challenges. Keywords: antisense oligonucleotides; siRNA; aptamers; DNAzymes; cancers Citation: Xiong, H.; Veedu, R.N.; 1. Introduction Diermeier, S.D. Recent Advances in Oligonucleotide Therapeutics in According to the Global Cancer Statistics 2018, there were more than 18 million new Oncology. Int. J. Mol. Sci. 2021, 22, cancer cases and 9.6 million deaths caused by cancer in 2018 [1]. -

Developmental Therapeutics Immunotherapy
DEVELOPMENTAL THERAPEUTICS—IMMUNOTHERAPY 2500 Oral Abstract Session Clinical activity of systemic VSV-IFNb-NIS oncolytic virotherapy in patients with relapsed refractory T-cell lymphoma. Joselle Cook, Kah Whye Peng, Susan Michelle Geyer, Brenda F. Ginos, Amylou C. Dueck, Nandakumar Packiriswamy, Lianwen Zhang, Beth Brunton, Baskar Balakrishnan, Thomas E. Witzig, Stephen M Broski, Mrinal Patnaik, Francis Buadi, Angela Dispenzieri, Morie A. Gertz, Leif P. Bergsagel, S. Vincent Rajkumar, Shaji Kumar, Stephen J. Russell, Martha Lacy; Mayo Clinic, Rochester, MN; The Ohio State University, Columbus, OH; Mayo Clinic, Scottsdale, AZ; Division of Hematology, Mayo Clinic, Roches- ter, MN; Vyriad and Mayo Clinic, Rochester, MN Background: Oncolytic virotherapy is a novel immunomodulatory therapeutic approach for relapsed re- fractory hematologic malignancies. The Indiana strain of Vesicular Stomatitis Virus was engineered to encode interferon beta (IFNb) and sodium iodine symporter (NIS) to produce VSV-IFNb-NIS. Virally en- coded IFNb serves as an index of viral proliferation and enhances host anti-tumor immunity. NIS was in- serted to noninvasively assess viral biodistribution using SPECT/PET imaging. We present the results of the phase 1 clinical trial NCT03017820 of systemic administration of VSV-IFNb-NIS among patients (pts) with relapsed refractory Multiple Myeloma (MM), T cell Lymphoma (TCL) and Acute myeloid Leu- 9 kemia (AML). Methods: VSV-IFNb-NIS was administered at 5x10 TCID50 (50% tissue culture infec- 11 tious dose) dose level 1 to dose level 4, 1.7x10 TCID50. The primary objective was to determine the maximum tolerated dose of VSV-IFNb-NIS as a single agent. Secondary objectives were determination of safety profile and preliminary efficacy of VSV-IFNb-NIS. -

2020 Medicaid Preapproval Criteria
2020 Medicaid Preapproval Criteria ABILIFY MAINTENA ................................................................................................................................................................ 10 ACTHAR HP ............................................................................................................................................................................ 11 ACTIMMUNE ......................................................................................................................................................................... 13 ADCIRCA ................................................................................................................................................................................ 14 ADEMPAS .............................................................................................................................................................................. 15 ADENOSINE DEAMINASE (ADA) REPLACEMENT ................................................................................................................... 17 AFINITOR ............................................................................................................................................................................... 18 AFINITOR DISPERZ ................................................................................................................................................................. 19 ALDURAZYME ....................................................................................................................................................................... -
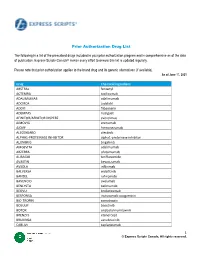
ESC Prior Authorization Program Drug List
Prior Authorization Drug List The following is a list of the prescribed drugs included in your prior authorization program and is comprehensive as of the date of publication. Express Scripts Canada® makes every effort to ensure this list is updated regularly. Please note that prior authorization applies to the brand drug and its generic alternatives (if available). As of June 17, 2021 Drug Chemical ingredient ABSTRAL fentanyl ACTEMRA tocilizumab ADALIMUMAB adalimumab ADCIRCA tadalafil ADDYI flibanserin ADEMPAS riociguat AFINITOR/AFINITOR DISPERZ everolimus AIMOVIG erenumab AJOVY fremanezumab ALECENSARO alectinib ALPHA1-PROTEINASE INHIBITOR alpha1-proteinase inhibitor ALUNBRIG brigatinib AMGEVITA adalimumab ARZERRA ofatumumab AUBAGIO teriflunomide AVASTIN bevacizumab AVSOLA infliximab BALVERSA erdafitinib BANZEL rufinamide BAVENCIO avelumab BENLYSTA belimumab BEOVU brolucizumab BESPONSA inotuzumab ozogamicin BIO-TROPIN somatropin BOSULIF bosutinib BOTOX onabotulinumtoxinA BRENZYS etanercept BRUKINSA zanubrutinib CABLIVI caplacizumab 1 © Express Scripts Canada. All rights reserved. Drug Chemical ingredient CABOMETYX cabozantinib CALQUENCE acalabrutinib CAPRELSA vandetanib CARBAGLU carglumic acid CERDELGA eliglustat tartrate CIMZIA certolizumab CINQAIR reslizumab COPAXONE Glatiramer acetate COSENTYX secukinumab COTELLIC cobimetinib CRESEMBA isavuconazonium sulfate CRYSVITA burosumab CUVPOSA glycopyrrolate CYRAMZA ramucirumab CYSTADROPS cysteamine hcl DACOGEN decitabine DAKLINZA daclatasvir DAURISMO glasdegib DEMYLOCAN decitabine DIACOMIT