Molecular Phylogeny and Diversification History of Prosopis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
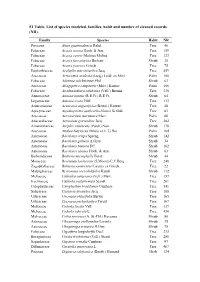
S1 Table. List of Species Modeled, Families, Habit and Number of Cleaned Records (NR)
S1 Table. List of species modeled, families, habit and number of cleaned records (NR). Family Species Habit NR Pinaceae Abies guatemalensis Rehd. Tree 46 Fabaceae Acacia aroma Hook. & Arn. Tree 159 Fabaceae Acacia caven (Molina) Molina Tree 123 Fabaceae Acacia furcatispina Burkart Shrub 35 Fabaceae Acacia praecox Griseb. Tree 75 Euphorbiaceae Acalypha macrostachya Jacq. Tree 459 Arecaceae Acrocomia aculeata (Jacq.) Lodd. ex Mart. Palm 166 Fabaceae Adesmia volckmannii Phil. Shrub 63 Arecaceae Allagoptera campestris (Mart.) Kuntze Palm 106 Fabaceae Anadenanthera colubrina (Vell.) Brenan Tree 330 Annonaceae Annona nutans (R.E.Fr.) R.E.Fr. Shrub 64 Loganiaceae Antonia ovata Pohl Tree 113 Araucariaceae Araucaria angustifolia (Bertol.) Kuntze Tree 48 Apocynaceae Aspidosperma quebracho-blanco Schltdl. Tree 83 Arecaceae Astrocaryum murumuru Mart. Palm 66 Anacardiaceae Astronium graveolens Jacq. Tree 282 Amaranthaceae Atriplex canescens (Pursh) Nutt. Shrub 178 Arecaceae Attalea butyracea (Mutis ex L.f.) We Palm 104 Asteraceae Baccharis crispa Spreng. Shrub 142 Asteraceae Baccharis gilliesii A.Gray Shrub 34 Asteraceae Baccharis trimera DC. Shrub 162 Asteraceae Baccharis ulicina Hook. & Arn. Shrub 63 Berberidaceae Berberis microphylla Forst. Shrub 44 Moraceae Brosimum lactescens (S.Moore) C.C.Berg Tree 248 Zygophyllaceae Bulnesia sarmientoi Lorentz ex Griseb. Tree 22 Malpighiaceae Byrsonima coccolobifolia Kunth Shrub 112 Meliaceae Cabralea canjerana (Vell.) Mart. Tree 157 Icacinaceae Calatola costaricensis Standl. Tree 201 Calophyllaceae Calophyllum brasiliense Cambess. Tree 541 Salicaceae Casearia decandra Jacq. Tree 188 Urticaceae Cecropia obtusifolia Bertol. Tree 165 Urticaceae Cecropia pachystachya Trécul Tree 167 Meliaceae Cedrela fissilis Vell. Tree 137 Meliaceae Cedrela odorata L. Tree 436 Malvaceae Ceiba speciosa (A. St.-Hil.) Ravenna Shrub 80 Asteraceae Chuquiraga avellanedae Lorentz Shrub 35 Asteraceae Chuquiraga erinacea D.Don Shrub 75 Fabaceae Copaifera langsdorffii Desf. -

Supplementary Materialsupplementary Material
10.1071/BT13149_AC © CSIRO 2013 Australian Journal of Botany 2013, 61(6), 436–445 SUPPLEMENTARY MATERIAL Comparative dating of Acacia: combining fossils and multiple phylogenies to infer ages of clades with poor fossil records Joseph T. MillerA,E, Daniel J. MurphyB, Simon Y. W. HoC, David J. CantrillB and David SeiglerD ACentre for Australian National Biodiversity Research, CSIRO Plant Industry, GPO Box 1600 Canberra, ACT 2601, Australia. BRoyal Botanic Gardens Melbourne, Birdwood Avenue, South Yarra, Vic. 3141, Australia. CSchool of Biological Sciences, Edgeworth David Building, University of Sydney, Sydney, NSW 2006, Australia. DDepartment of Plant Biology, University of Illinois, Urbana, IL 61801, USA. ECorresponding author. Email: [email protected] Table S1 Materials used in the study Taxon Dataset Genbank Acacia abbreviata Maslin 2 3 JF420287 JF420065 JF420395 KC421289 KC796176 JF420499 Acacia adoxa Pedley 2 3 JF420044 AF523076 AF195716 AF195684; AF195703 Acacia ampliceps Maslin 1 KC421930 EU439994 EU811845 Acacia anceps DC. 2 3 JF420244 JF420350 JF419919 JF420130 JF420456 Acacia aneura F.Muell. ex Benth 2 3 JF420259 JF420036 JF420366 JF419935 JF420146 KF048140 Acacia aneura F.Muell. ex Benth. 1 2 3 JF420293 JF420402 KC421323 JQ248740 JF420505 Acacia baeuerlenii Maiden & R.T.Baker 2 3 JF420229 JQ248866 JF420336 JF419909 JF420115 JF420448 Acacia beckleri Tindale 2 3 JF420260 JF420037 JF420367 JF419936 JF420147 JF420473 Acacia cochlearis (Labill.) H.L.Wendl. 2 3 KC283897 KC200719 JQ943314 AF523156 KC284140 KC957934 Acacia cognata Domin 2 3 JF420246 JF420022 JF420352 JF419921 JF420132 JF420458 Acacia cultriformis A.Cunn. ex G.Don 2 3 JF420278 JF420056 JF420387 KC421263 KC796172 JF420494 Acacia cupularis Domin 2 3 JF420247 JF420023 JF420353 JF419922 JF420133 JF420459 Acacia dealbata Link 2 3 JF420269 JF420378 KC421251 KC955787 JF420485 Acacia dealbata Link 2 3 KC283375 KC200761 JQ942686 KC421315 KC284195 Acacia deanei (R.T.Baker) M.B.Welch, Coombs 2 3 JF420294 JF420403 KC421329 KC955795 & McGlynn JF420506 Acacia dempsteri F.Muell. -

Allopolyploidy and Root Nodule Symbiosis in Glycine
TWO TO TANGO: ALLOPOLYPLOIDY AND ROOT NODULE SYMBIOSIS IN GLYCINE SUBGENUS GLYCINE A Dissertation Presented to the Faculty of the Graduate School of Cornell University in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy by Adrian Federico Powell January 2017 © 2017 Adrian Federico Powell TWO TO TANGO: ALLOPOLYPLOIDY AND ROOT NODULE SYMBIOSIS IN GLYCINE SUBGENUS GLYCINE Adrian Federico Powell, Ph.D. Cornell University 2017 Polyploidy (or whole genome duplication) and root nodule symbioses with bacteria (termed ‘rhizobia’) have both been important phenomena in the evolutionary history of the legume family (Leguminosae). Recently, it has been proposed that polyploidy may have played a critical role in the development or refinement of nodulation. Given the varied potential impacts of polyploidy, effects on biotic interactions are plausible. However, direct studies of the interactions between these phenomena in symbiotic, nodule-forming species are lacking. In this dissertation, using a complex of recently formed allopolyploids in Glycine subgenus Glycine, the perennial relatives of soybean, we examined (1) the root metabolites and symbiotic signaling capacity of multiple allopolyploid species relative to the diploid progenitor species that hybridized to form each allopolyploid, (2) the nodulation-related responses of allopolyploids and diploid progenitors to rhizobia and (3) the transcriptome-level responses to inoculation in allopolyploid G. dolichocarpa (T2) and its diploid progenitors. These objectives were pursued using a variety of approaches including root metabolite profiling, inoculation trials, and RNA sequencing. We found that, while there were no common transgressive patterns in the root metabolite profiles of allopolyploids in the complex, one of the progenitors of T2 had distinctive root metabolite and exudate profiles; profiles of symbiotic signaling metabolites were also altered in the allopolyploid. -

The Invasion Ecology of Acacia Elata (A
The invasion ecology of Acacia elata (A. Cunn. Ex Benth.) with implications for the management of ornamental wattles by Jason Ernest Donaldson Thesis presented in fulfilment of the requirements for the degree of Masters in the Faculty of Science at Stellenbosch University Supervisor: Prof. David M. Richardson Co-supervisor: Dr. John R. Wilson December 2013 Stellenbosch University http://scholar.sun.ac.za Declaration By submitting this thesis electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated), that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights, and that I have not previously in its entirety or in part submitted it for obtaining any qualification. September 2013 Copyright © 2013 Stellenbosch University All rights reserved i Stellenbosch University http://scholar.sun.ac.za Abstract This thesis explores how human dictated methods of introduction and species-specific traits interact to define spatial patterns in invasive plant populations using Acacia elata as a model species. I initially asked whether the relatively small invasive extent (when compared to congeners introduced for forestry or dune stabilization) of a species used widely for ornamental purposes (A. elata) is due to low rates of reproduction in South Africa. Results indicate that A. elata has similar traits to other invasive Australia Acacia species: annual seed input into the leaf litter was high (up to 5000 seeds m-2); large seedbanks develop (>20 000 seeds m-2) in established stands; seed germinability is very high (>90%); seeds accumulate mostly in the top soil layers but can infiltrate to depths of 40cm; and seed germination appears to be stimulated by fire. -

Native Plants Sixth Edition Sixth Edition AUSTRALIAN Native Plants Cultivation, Use in Landscaping and Propagation
AUSTRALIAN NATIVE PLANTS SIXTH EDITION SIXTH EDITION AUSTRALIAN NATIVE PLANTS Cultivation, Use in Landscaping and Propagation John W. Wrigley Murray Fagg Sixth Edition published in Australia in 2013 by ACKNOWLEDGEMENTS Reed New Holland an imprint of New Holland Publishers (Australia) Pty Ltd Sydney • Auckland • London • Cape Town Many people have helped us since 1977 when we began writing the first edition of Garfield House 86–88 Edgware Road London W2 2EA United Kingdom Australian Native Plants. Some of these folk have regrettably passed on, others have moved 1/66 Gibbes Street Chatswood NSW 2067 Australia to different areas. We endeavour here to acknowledge their assistance, without which the 218 Lake Road Northcote Auckland New Zealand Wembley Square First Floor Solan Road Gardens Cape Town 8001 South Africa various editions of this book would not have been as useful to so many gardeners and lovers of Australian plants. www.newhollandpublishers.com To the following people, our sincere thanks: Steve Adams, Ralph Bailey, Natalie Barnett, www.newholland.com.au Tony Bean, Lloyd Bird, John Birks, Mr and Mrs Blacklock, Don Blaxell, Jim Bourner, John Copyright © 2013 in text: John Wrigley Briggs, Colin Broadfoot, Dot Brown, the late George Brown, Ray Brown, Leslie Conway, Copyright © 2013 in map: Ian Faulkner Copyright © 2013 in photographs and illustrations: Murray Fagg Russell and Sharon Costin, Kirsten Cowley, Lyn Craven (Petraeomyrtus punicea photograph) Copyright © 2013 New Holland Publishers (Australia) Pty Ltd Richard Cummings, Bert -

503 Flora V7 2.Doc 3
Browse LNG Precinct ©WOODSIDE Browse Liquefied Natural Gas Precinct Strategic Assessment Report (Draft for Public Review) December 2010 Appendix C-18 A Vegetation and Flora Survey of James Price Point: Wet Season 2009 A Vegetation and Flora Survey of James Price Point: Wet Season 2009 Prepared for Department of State Development December 2009 A Vegetation and Flora Survey of James Price Point: Wet Season 2009 © Biota Environmental Sciences Pty Ltd 2009 ABN 49 092 687 119 Level 1, 228 Carr Place Leederville Western Australia 6007 Ph: (08) 9328 1900 Fax: (08) 9328 6138 Project No.: 503 Prepared by: P. Chukowry, M. Maier Checked by: G. Humphreys Approved for Issue: M. Maier This document has been prepared to the requirements of the client identified on the cover page and no representation is made to any third party. It may be cited for the purposes of scientific research or other fair use, but it may not be reproduced or distributed to any third party by any physical or electronic means without the express permission of the client for whom it was prepared or Biota Environmental Sciences Pty Ltd. This report has been designed for double-sided printing. Hard copies supplied by Biota are printed on recycled paper. Cube:Current:503 (Kimberley Hub Wet Season):Doc:Flora:503 flora v7_2.doc 3 A Vegetation and Flora Survey of James Price Point: Wet Season 2009 4 Cube:Current:503 (Kimberley Hub Wet Season):Doc:Flora:503 flora v7_2.doc Biota A Vegetation and Flora Survey of James Price Point: Wet Season 2009 A Vegetation and Flora Survey of James Price -

Tree and Tree-Like Species of Mexico: Asteraceae, Leguminosae, and Rubiaceae
Revista Mexicana de Biodiversidad 84: 439-470, 2013 Revista Mexicana de Biodiversidad 84: 439-470, 2013 DOI: 10.7550/rmb.32013 DOI: 10.7550/rmb.32013439 Tree and tree-like species of Mexico: Asteraceae, Leguminosae, and Rubiaceae Especies arbóreas y arborescentes de México: Asteraceae, Leguminosae y Rubiaceae Martin Ricker , Héctor M. Hernández, Mario Sousa and Helga Ochoterena Herbario Nacional de México, Departamento de Botánica, Instituto de Biología, Universidad Nacional Autónoma de México. Apartado postal 70- 233, 04510 México D. F., Mexico. [email protected] Abstract. Trees or tree-like plants are defined here broadly as perennial, self-supporting plants with a total height of at least 5 m (without ascending leaves or inflorescences), and with one or several erect stems with a diameter of at least 10 cm. We continue our compilation of an updated list of all native Mexican tree species with the dicotyledonous families Asteraceae (36 species, 39% endemic), Leguminosae with its 3 subfamilies (449 species, 41% endemic), and Rubiaceae (134 species, 24% endemic). The tallest tree species reach 20 m in the Asteraceae, 70 m in the Leguminosae, and also 70 m in the Rubiaceae. The species-richest genus is Lonchocarpus with 67 tree species in Mexico. Three legume genera are endemic to Mexico (Conzattia, Hesperothamnus, and Heteroflorum). The appendix lists all species, including their original publication, references of taxonomic revisions, existence of subspecies or varieties, maximum height in Mexico, and endemism status. Key words: biodiversity, flora, tree definition. Resumen. Las plantas arbóreas o arborescentes se definen aquí en un sentido amplio como plantas perennes que se pueden sostener por sí solas, con una altura total de al menos 5 m (sin considerar hojas o inflorescencias ascendentes) y con uno o varios tallos erectos de un diámetro de al menos 10 cm. -

BIODIVERSITY CONSERVATION on the TIWI ISLANDS, NORTHERN TERRITORY: Part 1. Environments and Plants
BIODIVERSITY CONSERVATION ON THE TIWI ISLANDS, NORTHERN TERRITORY: Part 1. Environments and plants Report prepared by John Woinarski, Kym Brennan, Ian Cowie, Raelee Kerrigan and Craig Hempel. Darwin, August 2003 Cover photo: Tall forests dominated by Darwin stringybark Eucalyptus tetrodonta, Darwin woollybutt E. miniata and Melville Island Bloodwood Corymbia nesophila are the principal landscape element across the Tiwi islands (photo: Craig Hempel). i SUMMARY The Tiwi Islands comprise two of Australia’s largest offshore islands - Bathurst (with an area of 1693 km 2) and Melville (5788 km 2) Islands. These are Aboriginal lands lying about 20 km to the north of Darwin, Northern Territory. The islands are of generally low relief with relatively simple geological patterning. They have the highest rainfall in the Northern Territory (to about 2000 mm annual average rainfall in the far north-west of Melville and north of Bathurst). The human population of about 2000 people lives mainly in the three towns of Nguiu, Milakapati and Pirlangimpi. Tall forests dominated by Eucalyptus miniata, E. tetrodonta, and Corymbia nesophila cover about 75% of the island area. These include the best developed eucalypt forests in the Northern Territory. The Tiwi Islands also include nearly 1300 rainforest patches, with floristic composition in many of these patches distinct from that of the Northern Territory mainland. Although the total extent of rainforest on the Tiwi Islands is small (around 160 km 2 ), at an NT level this makes up an unusually high proportion of the landscape and comprises between 6 and 15% of the total NT rainforest extent. The Tiwi Islands also include nearly 200 km 2 of “treeless plains”, a vegetation type largely restricted to these islands. -

Flora Survey on Hiltaba Station and Gawler Ranges National Park
Flora Survey on Hiltaba Station and Gawler Ranges National Park Hiltaba Pastoral Lease and Gawler Ranges National Park, South Australia Survey conducted: 12 to 22 Nov 2012 Report submitted: 22 May 2013 P.J. Lang, J. Kellermann, G.H. Bell & H.B. Cross with contributions from C.J. Brodie, H.P. Vonow & M. Waycott SA Department of Environment, Water and Natural Resources Vascular plants, macrofungi, lichens, and bryophytes Bush Blitz – Flora Survey on Hiltaba Station and Gawler Ranges NP, November 2012 Report submitted to Bush Blitz, Australian Biological Resources Study: 22 May 2013. Published online on http://data.environment.sa.gov.au/: 25 Nov. 2016. ISBN 978-1-922027-49-8 (pdf) © Department of Environment, Water and Natural Resouces, South Australia, 2013. With the exception of the Piping Shrike emblem, images, and other material or devices protected by a trademark and subject to review by the Government of South Australia at all times, this report is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. All other rights are reserved. This report should be cited as: Lang, P.J.1, Kellermann, J.1, 2, Bell, G.H.1 & Cross, H.B.1, 2, 3 (2013). Flora survey on Hiltaba Station and Gawler Ranges National Park: vascular plants, macrofungi, lichens, and bryophytes. Report for Bush Blitz, Australian Biological Resources Study, Canberra. (Department of Environment, Water and Natural Resources, South Australia: Adelaide). Authors’ addresses: 1State Herbarium of South Australia, Department of Environment, Water and Natural Resources (DEWNR), GPO Box 1047, Adelaide, SA 5001, Australia. -

Acacia in THIS ISSUE Dacacia the Name Acacia Comes This Issue of Seed Notes from the Greek Acacia, Ace Will Cover the Genus Or Acis Meaning a Point Or Acacia
No. 9 Acacia IN THIS ISSUE DAcacia The name Acacia comes This issue of Seed Notes from the Greek acacia, ace will cover the genus or acis meaning a point or Acacia. thorn, or from acazo, to D Description sharpen, although this name applies more to African than D Geographic Australian species (Australian distribution and Acacia have no thorns or habitat larger prickles, unlike those D Reproductive biology that are native to Africa). D Seed collection Many species of Acacia, or wattles as they are commonly D Phyllodes and flowers of Acacia aprica. Photo – Andrew Crawford Seed quality called in Australia, are valuable assessment for a range of uses, in D Seed germination particular as garden plants. Description In Australia, Acacia (family is modified to form a leaf- D Recommended reading They are also used for amenity plantings, windbreaks, shade DMimosaceae) are woody like structure or phyllode. trees, groundcovers, erosion plants that range from These phyllodes may be flat and salinity control. The timber prostrate under-shrubs to or terete. Some species do of some Acacia is very hard tall trees. Acacia flowers are not have phyllodes and the and is ideal for fence posts small, regular and usually flattened stems or cladodes (e.g. A. saligna or jam). Other bisexual. They occur in spikes act as leaves. Foliage can Acacia species are used to or in globular heads and vary from blueish to dark make furniture and ornaments. range in colour from cream green to silvery grey. Most The seed of some wattles is to intense yellow. The leaves species of Acacia have glands a good food source for birds, of Acacia may be bipinnate on the axis of the phyllodes, other animals and humans (the primary leaflets being although in Australian as ‘bush tucker’. -

The Value of Fringing Vegetation (Watercourse)
TheThe ValueValue ofof FringingFringing VegetationVegetation UnaUna BellBell Dedicated to the memory of Dr Luke J. Pen An Inspiration to Us All Acknowledgements This booklet is the result of a request from the Jane Brook Catchment Group for a booklet that focuses on the local native plants along creeks in Perth Hills. Thank you to the Jane Brook Catchment Group, Shire of Kalamunda, Environmental Advisory Committee of the Shire of Mundaring, Eastern Metropolitan Regional Council, Eastern Hills Catchment Management Program and Mundaring Community Bank Branch, Bendigo Bank who have all provided funding for this project. Without their support this project would not have come to fruition. Over the course of working on this booklet many people have helped in various ways. I particularly wish to thank past and present Catchment Officers and staff from the Shire of Kalamunda, the Shire of Mundaring and the EMRC, especially Shenaye Hummerston, Kylie del Fante, Renee d’Herville, Craig Wansbrough, Toni Burbidge and Ryan Hepworth, as well as Graham Zemunik, and members of the Jane Brook Catchment Group. I also wish to thank the WA Herbarium staff, especially Louise Biggs, Mike Hislop, Karina Knight and Christine Hollister. Booklet design - Rita Riedel, Shire of Kalamunda About the Author Una Bell has a BA (Social Science) (Hons.) and a Graduate Diploma in Landcare. She is a Research Associate at the WA Herbarium with an interest in native grasses, Community Chairperson of the Eastern Hills Catchment Management Program, a member of the Jane Brook Catchment Group, and has been a bush care volunteer for over 20 years. Other publications include Common Native Grasses of South-West WA. -

Low Flammability Local Native Species (Complete List)
Indicative List of Low Flammability Plants – All local native species – Shire of Serpentine Jarrahdale – May 2010 Low flammability local native species (complete list) Location key – preferred soil types for local native species Location Soil type Comments P Pinjarra Plain Beermullah, Guildford and Serpentine River soils Alluvial soils, fertile clays and loams; usually flat deposits carried down from the scarp Natural vegetation is typical of wetlands, with sheoaks and paperbarks, or marri and flooded gum woodlands, or shrublands, herblands or sedgelands B Bassendean Dunes Bassendean sands, Southern River and Bassendean swamps Pale grey-yellow sand, infertile, often acidic, lacking in organic matter Natural vegetation is banksia woodland with woollybush, or woodlands of paperbarks, flooded gum, marri and banksia in swamps F Foothills Forrestfield soils (Ridge Hill Shelf) Sand and gravel Natural vegetation is woodland of jarrah and marri on gravel, with banksias, sheoaks and woody pear on sand S Darling Scarp Clay-gravels, compacted hard in summer, moist in winter, prone to erosion on steep slopes Natural vegetation on shallow soils is shrublands, on deeper soils is woodland of jarrah, marri, wandoo and flooded gum D Darling Plateau Clay-gravels, compacted hard in summer, moist in winter Natural vegetation on laterite (gravel) is woodland or forest of jarrah and marri with banksia and snottygobble, on granite outcrops is woodland, shrubland or herbs, in valleys is forests of jarrah, marri, yarri and flooded gum with banksia Flammability