And Ring Ouzel (Turdus Torquatus) in Switzerland
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Hike the Swiss Alps 23Nd Annual | September 11-22, 2016
HIKE THE SWISS ALPS 23ND ANNUAL | SEPTEMBER 11-22, 2016 Guided by Virginia Van Der Veer & Terry De Wald Experience the Swiss Alps the best way of all – on foot with a small, congenial group of friends! Sponsored by Internationally-known Tanque Verde Ranch. Hiking Director, Virginia Van der Veer, and Terry DeWald, experienced mountaineer, lead the group limited to 15 guests. Having lived in Europe for many years, Virginia has in-depth knowledge of the customs of the people and places visited. She has experience guiding Alpine hiking tours and is fluent in German. Terry has mountaineering experience in the Alps and has guided hikers in Switzerland. INCLUDED IN PACKAGE… • Guided intermediate level day-hikes in spectacular scenery. • Opportunities for easy walks or more advanced hiking daily. • 5 nights hotel in Kandersteg, an alpine village paradise. • 1 day trip to Zermatt with views of the Matterhorn. • 5 nights hotel in Wengen with views of the Eiger and Jungfrau. • 2 nights in 4-star Swissotel, Zurich. • Hearty breakfast buffets daily. • 3 or 4-course dinners daily. • Swiss Rail Pass, allowing unlimited travel on Swiss railroads, lake streamers, PTT buses and city transports. • Day-trip to world-famous Zermatt at the foot of the Matterhorn. Opportunity for day-hike with views of the world’s most photographed mountain. • Visit to Lucerne. TOUR PRICING… Tour price $4,595(single supplement is $325 if required) Tour begins and ends in Zurich. A deposit of $800 is due at booking. Full payment is due at the Ranch by July 15. Early booking is advised due to small group size. -

European Red List of Birds 2015
Turdus torquatus (Ring Ouzel) European Red List of Birds Supplementary Material The European Union (EU27) Red List assessments were based principally on the official data reported by EU Member States to the European Commission under Article 12 of the Birds Directive in 2013-14. For the European Red List assessments, similar data were sourced from BirdLife Partners and other collaborating experts in other European countries and territories. For more information, see BirdLife International (2015). Contents Reported national population sizes and trends p. 2 Trend maps of reported national population data p. 4 Sources of reported national population data p. 6 Species factsheet bibliography p. 10 Recommended citation BirdLife International (2015) European Red List of Birds. Luxembourg: Office for Official Publications of the European Communities. Further information http://www.birdlife.org/datazone/info/euroredlist http://www.birdlife.org/europe-and-central-asia/european-red-list-birds-0 http://www.iucnredlist.org/initiatives/europe http://ec.europa.eu/environment/nature/conservation/species/redlist/ Data requests and feedback To request access to these data in electronic format, provide new information, correct any errors or provide feedback, please email [email protected]. THE IUCN RED LIST OF THREATENED SPECIES™ BirdLife International (2015) European Red List of Birds Turdus torquatus (Ring Ouzel) Table 1. Reported national breeding population size and trends in Europe1. Country (or Population estimate Short-term population trend4 Long-term -

Scottish Birds 22: 9-19
Scottish Birds THE JOURNAL OF THE SOC Vol 22 No 1 June 2001 Roof and ground nesting Eurasian Oystercatchers in Aberdeen The contrasting status of Ring Ouzels in 2 areas of upper Deeside The distribution of Crested Tits in Scotland during the 1990s Western Capercaillie captures in snares Amendments to the Scottish List Scottish List: species and subspecies Breeding biology of Ring Ouzels in Glen Esk Scottish Birds The Journal of the Scottish Ornithologists' Club Editor: Dr S da Prato Assisted by: Dr I Bainbridge, Professor D Jenkins, Dr M Marquiss, Dr J B Nelson, and R Swann Business Editor: The Secretary sac, 21 Regent Terrace Edinburgh EH7 5BT (tel 0131-5566042, fax 0131 5589947, email [email protected]). Scottish Birds, the official journal of the Scottish Ornithologists' Club, publishes original material relating to ornithology in Scotland. Papers and notes should be sent to The Editor, Scottish Birds, 21 Regent Terrace, Edinburgh EH7 SBT. Two issues of Scottish Birds are published each year, in June and in December. Scottish Birds is issued free to members of the Scottish Ornithologists' Club, who also receive the quarterly newsletter Scottish Bird News, the annual Scottish Bird Report and the annual Raplor round up. These are available to Institutions at a subscription rate (1997) of £36. The Scottish Ornithologists' Club was formed in 1936 to encourage all aspects of ornithology in Scotland. It has local branches which meet in Aberdeen, Ayr, the Borders, Dumfries, Dundee, Edinburgh, Glasgow, Inverness, New Galloway, Orkney, St Andrews, Stirling, Stranraer and Thurso, each with its own programme of field meetings and winter lectures. -

Stauverminderung Reichenbach Im Kandertal Bericht Zur Mitwirkung
Oberingenieurkreis I Ier arrondissement d'Ingénieur en chef Tiefbauamt Office des ponts et des Kantons Bern chaussées du canton de Berne Vorprojekt Strassen-Nr. 223 Revidiert Strassenzug Spiez - Frutigen - Kandersteg Projekt-Nr. 20023 / 14.014 Gemeinde Reichenbach im Kandertal Projekt vom 15.02.201220.12.2017 60 x 147 Bericht zur Mitwirkung Stauverminderung Reichenbach im Kandertal Projektverfassende LP Ingeneure AG B+S AG Laubeggstrasse 70 Weltpoststrasse 5 3000 Bern 31 3000 Bern 15 Tel. 031 359 40 40 Tel. 031 356 80 80 Gemeinde Reichenbach Stauverminderung Reichenbach im Kandertal– Mitwirkungsprojekt / Bericht zur Mitwirkung Verfasser, Impressum und Dokumentenverwaltung Verfasser Impressum Erstelldatum: 28.10.2017 letzte Änderung: 20.12.2017 Autoren: Marino Sansoni Auftragsnummer: B.14.014.02. Datei: H:\DAT\b_reiver\31_Vorproj\11_Mitwirkung\05_MW-Bericht - def. abgegeben\Be_2017_12_20_Mitwirkungbericht_DEF.doc Seitenzahl: 18 (ohne Beilagen) Dokumentenverwaltung Version Datum Autor Bemerkungen 02.11.17 SAM Entwurf an OIK I 09.11.17 SAM Vorabzug an Lenkungsausschuss, Freigabe durch LA 20.12.17 SAM Definitive Version an OIK I LP Ingenieure AG Seite I Bau Verkehr Projektmanagement 20.12.2017 Gemeinde Reichenbach Stauverminderung Reichenbach im Kandertal– Mitwirkungsprojekt / Bericht zur Mitwirkung Inhaltsverzeichnis Inhaltsverzeichnis 1 Problemstellung / Ausgangslage 1 2 Aufbau / Inhalt Bericht zur Mitwirkung 1 3 Die Mitwirkung 2 4 Auswertung der Fragebogen 3 5 Beurteilung der heutigen Situation 3 5.1 Vorbemerkungen zur Funktionsweise -

Verwaltungskreis Frutigen-Niedersimmental in Der Verwaltungsregion Oberland
VERWALTUNGSKREIS FRUTIGEN-NIEDERSIMMENTAL IN DER VERWALTUNGSREGION OBERLAND Der Verwaltungskreis Frutigen-Niedersimmental besteht seit dem 1. Januar 2010 und gehört zur Verwaltungsregion Oberland. Er umfasst eine Fläche von 785 km² und rund 40’000 ständige Einwohner innen und Einwohner verteilt auf 13 Gemeinden mit rund 40 öffentlich-rechtlichen Körperschaften (Burgergemeinden und -bäuerten, Schwellengemeinden, Kirchgemeinden). FREUNDLICH LÖSUNGSORIENTIERT BÜRGERNAH EFFIZIENT WOHLWOLLEND RATGEBEND Aufsichtsbehörde KOMPETENT • Gemeinden und öffentlich- VERTRAUT rechtliche Körperschaften Bewilligungsbehörde • Vormundschaftsrecht • Baubewilligungsverfahren • Koordination in ausserordentlichen • Gastgewerbe Lagen • Bodenrecht / • Aufsichtsrechtliche Anzeigen Grundstückverkäufe an Ausländer DIE BEREICHE UND Verwaltungsjustiz Ombudsfunktion AUFGABEN DER • Beschwerdeverfahren • Ansprechpartner für alle Fragen VERANTWORTLICHEN • Verständigung der Zentralverwaltung DES REGIERUNGS- STATTHALTERAMTES DIE ORGANISATION SEIT 2010 Regierungsstatthalter • Führung / Koordination • Entscheide / Einspracheverhandlungen • Ombudsperson • Bäuerliches Bodenrecht / ausserordentliche Lagen Stabsdienste • Bäuerliches Bodenrecht • Grundstückverkäufe an Ausländer • Stabsarbeit Stellvertretung des Regierungsstatthalters Bauen / Finanzen • Rechtsauskünfte • Baubewilligungsverfahren • Koordination Beschwerdeverfahren • Finanzen • Gemeindenaufsicht • Abstimmungen und Wahlen Kanzlei • Gastgewerbe • Inventar • Archiv • Vormundschaft ÜBERSICHT ÜBER DIE GEMEINDEN DES VERWALTUNGSKREISES -

Postfledging Survival, Movements, and Dispersal of Ring Ouzels (Turdus Torquatus) Author(S): Innes M
Postfledging Survival, Movements, and Dispersal of Ring Ouzels (Turdus torquatus) Author(s): Innes M. W. Sim , Sonja C. Ludwig , Murray C. Grant , Joanna L. Loughrey , Graham W. Rebecca , and Jane M. Reid Source: The Auk, 130(1):69-77. 2013. Published By: The American Ornithologists' Union URL: http://www.bioone.org/doi/full/10.1525/auk.2012.12008 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. The Auk 130(1):69−77, 2013 © The American Ornithologists’ Union, 2013. Printed in USA. POSTFLEDGING SURVIVAL, MOVEMENTS, AND DISPERSAL OF RING OUZELS (TURDUS TORQUATUS) INNES M. W. SIM,1,2,4 SONJA C. LUDWIG,1,5 MURRAY C. GRANT,1,6 JOANNA L. LOUGHREY,2,6 GRAHAM -

Developing Methods for the Field Survey and Monitoring of Breeding Short-Eared Owls (Asio Flammeus) in the UK: Final Report from Pilot Fieldwork in 2006 and 2007
BTO Research Report No. 496 Developing methods for the field survey and monitoring of breeding Short-eared owls (Asio flammeus) in the UK: Final report from pilot fieldwork in 2006 and 2007 A report to Scottish Natural Heritage Ref: 14652 Authors John Calladine, Graeme Garner and Chris Wernham February 2008 BTO Scotland School of Biological and Environmental Sciences, University of Stirling, Stirling, FK9 4LA Registered Charity No. SC039193 ii CONTENTS LIST OF TABLES................................................................................................................... iii LIST OF FIGURES ...................................................................................................................v LIST OF FIGURES ...................................................................................................................v LIST OF APPENDICES...........................................................................................................vi SUMMARY.............................................................................................................................vii EXECUTIVE SUMMARY ................................................................................................... viii CRYNODEB............................................................................................................................xii ACKNOWLEDGEMENTS....................................................................................................xvi 1. BACKGROUND AND AIMS...........................................................................................2 -
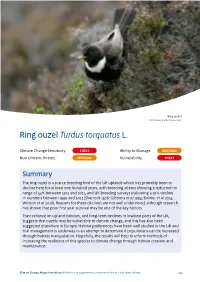
Climate Change Adaptation Manual Second Edition 2020
Ring ouzel © Andy Hay (rspb-images.com) Ring ouzel Turdus torquatus L. Climate Change Sensitivity: HIGH Ability to Manage: MEDIUM Non climatic threats: MEDIUM Vulnerability: HIGH Summary The ring ouzel is a scarce breeding bird of the UK uplands which has probably been in decline here for at least one hundred years, with breeding atlases showing a reduction in range of 44% between 1972 and 2011, and UK breeding surveys indicating a 29% decline in numbers between 1999 and 2012 (Sharrock 1976; Gibbons et al 1993; Balmer et al 2013; Wotton et al 2016). Reasons for these declines are not well understood, although research has shown that poor first year survival may be one of the key factors. Their reliance on upland habitats, and long-term declines in lowland parts of the UK, suggests that ouzels may be vulnerable to climate change, and this has also been suggested elsewhere in Europe. Habitat preferences have been well studied in the UK and trial management is underway in an attempt to determine if populations can be increased through habitat manipulation. Hopefully, the results will help to inform methods of increasing the resilience of this species to climate change through habitat creation and maintenance. Climate Change Adaptation Manual Evidence to support nature conservation in a changing climate 424 Description The ring ouzel is a medium-sized thrush, very similar to but slightly smaller and less stocky than a blackbird, and with slightly longer wings and a distinctive white-cream breast band or gorget in adults. Unlike blackbirds, ouzels have silvery panels in the wings and silver-grey edges to the lower body feathers, often giving them a scaly appearance, which fades with time following the annual moult. -

The Modern Sediments of Lake Oeschinen (Swiss Alps) As an Archive for Climatic and Meteorological Events
The modern sediments of Lake Oeschinen (Swiss Alps) as an archive for climatic and meteorological events Master’s Thesis Faculty of Science University of Bern presented by Fabian Mauchle 2010 Supervisor: Prof. Dr. Martin Grosjean Institute of Geography and Oeschger Centre for Climate Change Research Advisor: Dr. Mathias Trachsel Institute of Geography and Oeschger Centre for Climate Change Research II Lake Oeschinen, Fabian Mauchle, 2010 III IV Table of contents Table of contents Summary � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � XI Zusammenfassung � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � �XIII 1� Introduction � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � �1 1�1� Research motivation and general objectives � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 1 1�2� State of knowledge � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 2 1�3� Research questions and working hypothesis � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 3 1�4� Project design � � � � � � � � � � � � � � � � � � � � � � -

Population Trends of Common Birds in the Italian Alps
Population Index of Common Breeding Birds in Italian Mountain Prairies Rural development: A critical opportunity for people and biodiversity With a spotlight on the Alpine region Turin 6 november 2013 Mountains cover almost a quarter of the Earth surface, host more than 16% of global human population but provide services to many more communities and people Mountains provide many kind of resources that have positive impacts on human health and its prosperity far beyond their natural boundaries. One for all, water The mountains contribute for the 16% to the Italian national GDP However, despite their importance, the conservation status of mountains is not so satisfactory and many problems exist. Some of these are related with human presence and activities, e.g. effects of climate change, pollution, settlement and infrastructures development and tourism pressure; others with the opposite phenomena e.g. changes in cultural landscape and decrease of open habitats, and their biodiversity, due to the land abandonment For these reasons, MITO2000 project (the Italian national common bird monitoring scheme) decided to focus on species breeding in mountain open habitats MITO2000 is part of PECBMS (Pan-European Common Bird Monitoring Scheme), a Europe-wide network of national breeding bird monitoring projects. This network produces indices of population trends of many breeding bird species. One of the most important index is the FBI (Farmland Bird Index), that is one of the EU indicators to assess the impact of agri- environment measures of the CAP These indices are “aggregated indices” because they are calculated averaging the population trends of some species, choosen on the basis of their shared ecological preferences Through objective procedures we identified a group of species breeding in mountain open habitats and, from 2009, we used them to build up a new aggregate index, named FBIpm ( Mountain Prairies Index) Some results ..... -

Route Du Bonheur – Relais & Châteaux with Eurotrek World of Hiking Between Bernese Oberland and Valais Route Du Bonheur
Route du Bonheur – Relais & Châteaux with Eurotrek World of hiking between Bernese Oberland and Valais Route du Bonheur 6 days / 5 nights or 5 days / 4 nights Individual (selfguided) tour Eurotrek AG Zürcherstrasse 42, 8103 Unterengstringen Tel.: 044 316 10 00 | Fax.: 044 316 10 01 Mail: [email protected] | Web: www.eurotrek.ch Eurotrek: Route du Bonheur | Season 2018 | Version/Date: 12.04.2018 1/4 Route du Bonheur This hiking Route du Bonheur offers you an exclusive combination of extraordinary overnights in various Relais & Châteaux Hotels with as well extraodinary hiking experiences on carefully signposted routes in the Swiss Alps. Enjoy sparkling mountain lakes, magnificent views on snow-covered glaciers and visit charming mountain villages. A combination of adventure and a taste of luxury: welcome to the Route du Bonheur of Relais & Châteaux. Day-by-day Day 1: Arrival to Crans-Montana Overnight in Crans-Montana, at the Relais & Châteaux Hostellerie du Pas de l'Ours Day 2: Crans-Montana - Leukerbad Hike from Crans-Montana to Leukerbad on the well signposted Route "Walliser Sonnenweg". The stage from Crans-Montana to Leukerbad is rather long and demanding, but in return, Leukerbad welcomes weary hikers with warm thermal pools, Jacuzzis and other treats for body and soul. Cutting down of hiking times by partly using public transports is possible. Overnight in Leukerbad, at the Relais & Châteaux Hôtel Les Sources des Alpes Details: 24 km, ↑ 1'143 m ↓ 1'225 m, length: approx. 8.5 h Day 3: Cablecar Leukerbad – Gemmipass | Gemmipass – Kandersteg You may spare the 2 hours ascension from Leukerbad to Gemmipass and use the cable car. -

Übersicht Über Abgaben an Die Gemeinden
BKW POWER GRID Übersicht über Abgaben an die Gemeinden Abgabe Maximalbetrag Abgabe Maximalbetrag Gemeinde (Rp./kWh) pro Jahr (CHF) Gemeinde (Rp./kWh) pro Jahr (CHF) A Bonfol 1.50 300.00 Aarberg6 - - Bönigen 1.50 300.00 Aarwangen6 - - Bösingen6 - - Adelboden 1.50 300.00 Bourrignon 1.50 300.00 Aedermannsdorf6 - - Bowil1 1.50 300.00 Aeschi (SO)7 1.10 / 1.50 300.00 Bremgarten bei Bern1 1.50 300.00 Aeschi bei Spiez 1.50 300.00 Brenzikofen 1.50 300.00 Affoltern im Emmental 1.50 300.00 Brienz (BE)6 - - Alchenstorf 1.50 300.00 Brienzwiler6 - - Alle 1.50 300.00 Brislach6 - - Allmendingen 1.50 300.00 Brügg6 - - Amsoldingen 1.50 300.00 Brüttelen 1.50 300.00 Attiswil7 - / 1.50 - / 300.00 Buchholterberg 1.50 300.00 Auswil 1.50 300.00 Büetigen6 - - B Bühl 1.50 300.00 Balm bei Günsberg 1.10 300.00 Bure 1.50 300.00 Balsthal6 - - Burgdorf6 - - Bannwil 1.50 300.00 Burgistein 1.50 300.00 Basse-Allaine 1.50 300.00 Busswil bei Melchnau 1.50 300.00 Bätterkinden7 - / 1.50 - / 300.00 C Beatenberg 1.50 300.00 Champoz 1.50 300.00 Beinwil6 - - Châtillon (JU) 1.50 300.00 Bellach 1.10 300.00 Chevenez6 - - Bellmund6 - - Clos du Doubs 1.50 300.00 Belp 1.50 300.00 Coeuve 1.50 300.00 Belprahon 1.50 300.00 Corcelles (BE) 1.50 300.00 Berken 1.50 300.00 Corgémont 1.50 300.00 Bern6 - - Cornol 1.50 300.00 Bettenhausen 1.50 300.00 Courchapoix6 - - Bettlach 1.10 300.00 Courchavon 1.50 300.00 Beurnevésin 1.50 300.00 Courgenay 1.50 300.00 Biberist 1.00 2 000.00 Courrendlin 1.50 300.00 Biel (BE)6 - - Courroux 1.50 300.00 Biglen6 - - Court 1.50 300.00 Blauen 1.50 300.00 Courtedoux