Redox State During Core Formation on Asteroid 4-Vesta
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Laboratory Spectroscopy of Meteorite Samples at UV-Vis-NIR Wavelengths: Analysis and Discrimination by Principal Components Analysis
Laboratory spectroscopy of meteorite samples at UV-Vis-NIR wavelengths: Analysis and discrimination by principal components analysis Antti Penttil¨aa,∗, Julia Martikainena, Maria Gritsevicha, Karri Muinonena,b aDepartment of Physics, P.O. Box 64, FI-00014 University of Helsinki, Finland bFinnish Geospatial Research Institute FGI, National Land Survey of Finland, Geodeetinrinne 2, FI-02430 Masala, Finland Abstract Meteorite samples are measured with the University of Helsinki integrating-sphere UV-Vis- NIR spectrometer. The resulting spectra of 30 meteorites are compared with selected spectra from the NASA Planetary Data System meteorite spectra database. The spectral measure- ments are transformed with the principal component analysis, and it is shown that different meteorite types can be distinguished from the transformed data. The motivation is to im- prove the link between asteroid spectral observations and meteorite spectral measurements. Keywords: Meteorites, spectroscopy, principal component analysis 1. Introduction While a planet orbits the Sun, it is subject to impacts by objects ranging from tiny dust particles to much larger asteroids and comet nuclei. Such collisions of small Solar System bodies with planets have taken place frequently over geological time and played an 5 important role in the evolution of planets and development of life on the Earth. Every day approximately 30{180 tons of interplanetary material enter the Earth's atmosphere [1, 2]. This material is mostly represented by smaller meteoroids that undergo rapid ablation in the atmosphere. Under favorable initial conditions part of a meteoroid may survive the atmospheric entry and reach the ground [3]. The fragments recovered on the ground are 10 called meteorites, our valuable samples of the Solar System. -

N Arieuican%Mllsellm
n ARieuican%Mllsellm PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK 24, N.Y. NUMBER 2I63 DECEMBER I9, I963 The Pallasites BY BRIAN MASON' INTRODUCTION The pallasites are a comparatively rare type of meteorite, but are remarkable in several respects. Historically, it was a pallasite for which an extraterrestrial origin was first postulated because of its unique compositional and structural features. The Krasnoyarsk pallasite was discovered in 1749 about 150 miles south of Krasnoyarsk, and seen by P. S. Pallas in 1772, who recognized these unique features and arranged for its removal to the Academy of Sciences in St. Petersburg. Chladni (1794) examined it and concluded it must have come from beyond the earth, at a time when the scientific community did not accept the reality of stones falling from the sky. Compositionally, the combination of olivine and nickel-iron in subequal amounts clearly distinguishes the pallasites from all other groups of meteorites, and the remarkable juxtaposition of a comparatively light silicate mineral and heavy metal poses a nice problem of origin. Several theories of the internal structure of the earth have postulated the presence of a pallasitic layer to account for the geophysical data. No apology is therefore required for an attempt to provide a comprehensive account of this remarkable group of meteorites. Some 40 pallasites are known, of which only two, Marjalahti and Zaisho, were seen to fall (table 1). Of these, some may be portions of a single meteorite. It has been suggested that the pallasite found in Indian mounds at Anderson, Ohio, may be fragments of the Brenham meteorite, I Chairman, Department of Mineralogy, the American Museum of Natural History. -

Infrared Reflectance Spectra of Heds and Carbonaceous Chondrites
44th Lunar and Planetary Science Conference (2013) 1276.pdf KEYS TO DETECT SPACE WEATHERING ON VESTA: CHANGES OF VISIBLE AND NEAR- INFRARED REFLECTANCE SPECTRA OF HEDS AND CARBONACEOUS CHONDRITES. T. Hiroi1, S. Sasaki2, T. Misu3, and T. Nakamura3, 1Department of Geological Sciences, Brown University, Providence, RI 02912, USA ([email protected]), 2RISE Project, .National Astronomical Observatory of Japan, 2-12 Hoshigaoka-cho, Mizusawa-ku, Oshu, Iwate 023-0861, Japan, 3Department of Earth and Planetary Materials Science, Faculty of Science, Tohoku University, Aramaki, Aoba, Sendai, Miyagi 980-8578, Japan. Introduction: Space weathering is known to change the visible and near-infrared (VNIR) reflectance spectra of asteroidal surfaces. Past studies clearly showed the dependency of the rate of space weathering on iron content or crystal structure [1, 2]. In that respect, it is supposed that HED meteorite parent bodies would be very hard to space-weather, and space weathering of carbonaceous chondrite (CC) parent bodies would be very different from that of S- type asteroids [3]. Investigated in this study are key features useful for detecting space weathering in the VNIR spectra of HED and CC parent bodies. Experimental: Powder samples of CCs (<125 µm): Orgueil-Ivuna mixture (CI1), MAC 88100 (CM2), ALH 83108 (CO3), and ALH 85002 (CK4), and howardite EET 87503 (<25 m) were pressed into pellets, and their VNIR reflectance spectra (0.3-2.5 µm) were measured. The pellets were irradiated with pulse laser with energies of 5 and 10 mJ for the CC pellets and 75 mJ for the howardite pellet according to the procedure in [4], and their VNIR reflectance spectra were measured after each irradiation. -

Closing in on HED Meteorite Sources
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Springer - Publisher Connector Earth Planets Space, 53, 1077–1083, 2001 Closing in on HED meteorite sources Mark V. Sykes1 and Faith Vilas2 1Steward Observatory, University of Arizona, Tucson, Arizona 85721, U.S.A. 2NASA Johnson Space Center, SN3, Houston, Texas 77058, U.S.A. (Received March 25, 2001; Revised June 1, 2001; Accepted June 28, 2001) Members of the Vesta dynamical family have orbital elements consistent with ejecta from a single large exca- vating collision from a single hemisphere of Vesta. The portion of Vesta’s orbit at which such an event must have occurred is slightly constrained and depends on the hemisphere impacted. There is some evidence for subsequent disruptions of these ejected objects. Spectroscopy suggests that the dynamical family members are associated with material from Vesta’s interior that was excavated by the event giving rise to the crater covering much of Vesta’s southern hemisphere. The 505-nm pyroxene feature seen in HED meteorites is relatively absent in a sample of Vesta dynamical family members raising the question of whether this collisional event was the source of the HED meteorites. Asteroids spectroscopically associated with Vesta that possess this feature are dynamically distinct and could have arisen from a different collisional event on Vesta or originated from a body geochemically similar to Vesta that was disrupted early in the history of the solar system. 1. Introduction discovery of asteroid families by Hirayama (1918), such dy- The howardite-eucrite-diogenite (HED) meteorites com- namical clusters of objects have been thought to arise from prise ∼6% of present meteorite falls (McSween, 1999). -

Expanding Band Parameter Analysis Methods for HED Meteorites and V-Type Asteroids
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 5-2021 Expanding Band Parameter Analysis Methods for HED Meteorites and V-type Asteroids Noah Adm Haverkamp Frere University of Tennessee, Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Physical Processes Commons, and the The Sun and the Solar System Commons Recommended Citation Frere, Noah Adm Haverkamp, "Expanding Band Parameter Analysis Methods for HED Meteorites and V- type Asteroids. " Master's Thesis, University of Tennessee, 2021. https://trace.tennessee.edu/utk_gradthes/6217 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Noah Adm Haverkamp Frere entitled "Expanding Band Parameter Analysis Methods for HED Meteorites and V-type Asteroids." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Master of Science, with a major in . Sean S. Lindsay, Major Professor We have read this thesis and recommend its acceptance: Andrew W. Steiner, Tony Mezzacappa Accepted for the Council: Dixie L. Thompson Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) Expanding Band Parameter Analysis Methods for HED Meteorites and V-type Asteroids A Thesis Presented for the Master of Science Degree The University of Tennessee, Knoxville Noah Adm Haverkamp Frere May 2021 Copyright © by Noah Adm Haverkamp Frere, 2021 All Rights Reserved. -

Trace Element Chemistry of Cumulus Ridge 04071 Pallasite with Implications for Main Group Pallasites
Trace element chemistry of Cumulus Ridge 04071 pallasite with implications for main group pallasites Item Type Article; text Authors Danielson, L. R.; Righter, K.; Humayun, M. Citation Danielson, L. R., Righter, K., & Humayun, M. (2009). Trace element chemistry of Cumulus Ridge 04071 pallasite with implications for main group pallasites. Meteoritics & Planetary Science, 44(7), 1019-1032. DOI 10.1111/j.1945-5100.2009.tb00785.x Publisher The Meteoritical Society Journal Meteoritics & Planetary Science Rights Copyright © The Meteoritical Society Download date 23/09/2021 14:17:54 Item License http://rightsstatements.org/vocab/InC/1.0/ Version Final published version Link to Item http://hdl.handle.net/10150/656592 Meteoritics & Planetary Science 44, Nr 7, 1019–1032 (2009) Abstract available online at http://meteoritics.org Trace element chemistry of Cumulus Ridge 04071 pallasite with implications for main group pallasites Lisa R. DANIELSON1*, Kevin RIGHTER2, and Munir HUMAYUN3 1Mailcode JE23, NASA Johnson Space Center, 2101 NASA Parkway, Houston, Texas 77058, USA 2Mailcode KT, NASA Johnson Space Center, 2101 NASA Parkway, Houston, Texas 77058, USA 3National High Magnetic Field Laboratory and Department of Geological Sciences, Florida State University, Tallahassee, Florida 32310, USA *Corresponding author. E-mail: [email protected] (Received 06 November 2008; revision accepted 11 May 2009) Abstract–Pallasites have long been thought to represent samples from the metallic core–silicate mantle boundary of a small asteroid-sized body, with as many as ten different parent bodies recognized recently. This report focuses on the description, classification, and petrogenetic history of pallasite Cumulus Ridge (CMS) 04071 using electron microscopy and laser ablation ICP-MS. -
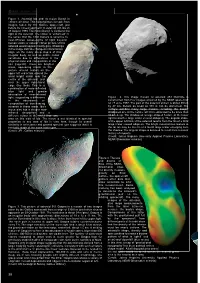
Iso and Asteroids
r bulletin 108 Figure 1. Asteroid Ida and its moon Dactyl in enhanced colour. This colour picture is made from images taken by the Galileo spacecraft just before its closest approach to asteroid 243 Ida on 28 August 1993. The moon Dactyl is visible to the right of the asteroid. The colour is ‘enhanced’ in the sense that the CCD camera is sensitive to near-infrared wavelengths of light beyond human vision; a ‘natural’ colour picture of this asteroid would appear mostly grey. Shadings in the image indicate changes in illumination angle on the many steep slopes of this irregular body, as well as subtle colour variations due to differences in the physical state and composition of the soil (regolith). There are brighter areas, appearing bluish in the picture, around craters on the upper left end of Ida, around the small bright crater near the centre of the asteroid, and near the upper right-hand edge (the limb). This is a combination of more reflected blue light and greater absorption of near-infrared light, suggesting a difference Figure 2. This image mosaic of asteroid 253 Mathilde is in the abundance or constructed from four images acquired by the NEAR spacecraft composition of iron-bearing on 27 June 1997. The part of the asteroid shown is about 59 km minerals in these areas. Ida’s by 47 km. Details as small as 380 m can be discerned. The moon also has a deeper near- surface exhibits many large craters, including the deeply infrared absorption and a shadowed one at the centre, which is estimated to be more than different colour in the violet than any 10 km deep. -

Download Version of Record (PDF / 6MB)
Open Research Online The Open University’s repository of research publications and other research outputs An Isotopic Investigation Of Early Planetesimal Differentiation Processes Thesis How to cite: Windmill, Richard Joseph (2021). An Isotopic Investigation Of Early Planetesimal Differentiation Processes. PhD thesis The Open University. For guidance on citations see FAQs. c 2020 Richard Joseph Windmill https://creativecommons.org/licenses/by-nc-nd/4.0/ Version: Version of Record Link(s) to article on publisher’s website: http://dx.doi.org/doi:10.21954/ou.ro.00012472 Copyright and Moral Rights for the articles on this site are retained by the individual authors and/or other copyright owners. For more information on Open Research Online’s data policy on reuse of materials please consult the policies page. oro.open.ac.uk AN ISOTOPIC INVESTIGATION OF EARLY PLANETESIMAL DIFFERENTIATION PROCESSES Richard J. Windmill Supervisors: Dr. I. A. Franchi Professor M. Anand Dr. R. C. Greenwood Submitted to the School of Physical Sciences at The Open University in accordance with the requirements for the degree of Doctor of Philosophy June 2020 School of Physical Sciences Robert Hooke Building The Open University Walton Hall Milton Keynes MK7 6AA United Kingdom Abstract The differentiation and early evolution of planetesimals is relatively poorly understood. The Main- Group pallasites (PMGs) and IIIAB irons are differentiated meteorite groups from deep planetesimal interiors. They provide a window into the early evolution of rocky planets because of the abundance of samples from these groups and because a common planetary provenance has been proposed. Oxygen isotope analyses are crucial in understanding these relationships. -

Vesta and the HED Meteorites: Mid-Infrared Modeling of Minerals and Their Abundances
Vesta and the HED meteorites: Mid-infrared modeling of minerals and their abundances Item Type Article; text Authors Donaldson Hanna, K.; Sprague, A. L. Citation Donaldson Hanna, K., & Sprague, A. L. (2009). Vesta and the HED meteorites: Midinfrared modeling of minerals and their abundances. Meteoritics & Planetary Science, 44(11), 1755-1770. DOI 10.1111/j.1945-5100.2009.tb01205.x Publisher The Meteoritical Society Journal Meteoritics & Planetary Science Rights Copyright © The Meteoritical Society Download date 24/09/2021 12:48:06 Item License http://rightsstatements.org/vocab/InC/1.0/ Version Final published version Link to Item http://hdl.handle.net/10150/656640 Meteoritics & Planetary Science 44, Nr 11, 1755–1770 (2009) Abstract available online at http://meteoritics.org Vesta and the HED meteorites: Mid-infrared modeling of minerals and their abundances Kerri DONALDSON HANNA1, 2* and Ann L. SPRAGUE1 1Lunar and Planetary Laboratory, University of Arizona, 1629 E. University Blvd., Tucson, Arizona 85721–0092, USA 2Present address: Department of Geological Sciences, Brown University, Box 1846, Providence, Rhode Island 02912, USA *Corresponding author. E-mail: [email protected] (Received 29 October 2007; revision accepted 12 August 2009) Abstract–We demonstrate that the use of an established spectral deconvolution algorithm with mid- infrared spectral libraries of mineral separates of varying grain sizes is capable of identifying the known mineral compositions and abundances of a selection of howardite, eucrite, and diogenite (HED) meteorite samples. In addition, we apply the same technique to mid-infrared spectral emissivity measurements of Vesta that have been obtained from Cornell’s Mid-Infrared Asteroid Spectroscopy (MIDAS) Survey and the Infrared Space Observatory (ISO). -

Transneptunian Object Taxonomy 181
Fulchignoni et al.: Transneptunian Object Taxonomy 181 Transneptunian Object Taxonomy Marcello Fulchignoni LESIA, Observatoire de Paris Irina Belskaya Kharkiv National University Maria Antonietta Barucci LESIA, Observatoire de Paris Maria Cristina De Sanctis IASF-INAF, Rome Alain Doressoundiram LESIA, Observatoire de Paris A taxonomic scheme based on multivariate statistics is proposed to distinguish groups of TNOs having the same behavior concerning their BVRIJ colors. As in the case of asteroids, the broadband spectrophotometry provides a first hint about the bulk compositional properties of the TNOs’ surfaces. Principal components (PC) analysis shows that most of the TNOs’ color variability can be accounted for by a single component (i.e., a linear combination of the col- ors): All the studied objects are distributed along a quasicontinuous trend spanning from “gray” (neutral color with respect to those of the Sun) to very “red” (showing a spectacular increase in the reflectance of the I and J bands). A finer structure is superimposed to this trend and four homogeneous “compositional” classes emerge clearly, and independently from the PC analy- sis, if the TNO sample is analyzed with a grouping technique (the G-mode statistics). The first class (designed as BB) contains the objects that are neutral in color with respect to the Sun, while the RR class contains the very red ones. Two intermediate classes are separated by the G mode: the BR and the IR, which are clearly distinguished by the reflectance relative increases in the R and I bands. Some characteristics of the classes are deduced that extend to all the objects of a given class the properties that are common to those members of the class for which more detailed data are available (observed activity, full spectra, albedo). -

105. Titanium Stable Isotopic Variations in Chondrites
Available online at www.sciencedirect.com ScienceDirect Geochimica et Cosmochimica Acta 213 (2017) 534–552 www.elsevier.com/locate/gca Titanium stable isotopic variations in chondrites, achondrites and lunar rocks Nicolas D. Greber a,⇑, Nicolas Dauphas a, Igor S. Puchtel b, Beda A. Hofmann c, Nicholas T. Arndt d a Origins Laboratory, Department of the Geophysical Sciences and Enrico Fermi Institute, The University of Chicago, 5734 South Ellis Avenue, Chicago, IL 60615, USA b Department of Geology, University of Maryland, College Park, MD 20742, USA c Naturhistorisches Museum der Burgergemeinde Bern, Bernastrasse 15, Bern, Switzerland d Universite´ Grenoble Alpes, Institute Science de la Terre (ISTerre), CNRS, F-38041 Grenoble, France Received 22 December 2016; accepted in revised form 21 June 2017; Available online 30 June 2017 Abstract Titanium isotopes are potential tracers of processes of evaporation/condensation in the solar nebula and magmatic differ- entiation in planetary bodies. To gain new insights into the processes that control Ti isotopic variations in planetary materials, 25 komatiites, 15 chondrites, 11 HED-clan meteorites, 5 angrites, 6 aubrites, a martian shergottite, and a KREEP-rich impact melt breccia have been analyzed for their mass-dependent Ti isotopic compositions, presented using the d49Ti notation (devi- ation in permil of the 49Ti/47Ti ratio relative to the OL-Ti standard). No significant variation in d49Ti is found among ordi- nary, enstatite, and carbonaceous chondrites, and the average chondritic d49Ti value of +0.004 ± 0.010‰ is in excellent agreement with the published estimate for the bulk silicate Earth, the Moon, Mars, and the HED and angrite parent- bodies. -

Physical Modeling for the Vis-SWIR Spectrometry of the Chelyabinsk Meteorite
Master’s thesis Astronomy Physical modeling for the Vis-SWIR spectrometry of the Chelyabinsk meteorite Julia Martikainen August 22, 2015 Tutors: Dr. Antti Penttilä and Prof. Karri Muinonen Censors: Prof. Karri Muinonen Dr. Tomas Kohout UNIVERSITY OF HELSINKI Department of Physics PL 64 (Gustaf Hällströmin katu 2) 00014 Helsingin yliopisto “Once we accept our limits, we go beyond them.” —Albert Einstein HELSINGIN YLIOPISTO — HELSINGFORS UNIVERSITET — UNIVERSITY OF HELSINKI Tiedekunta — Fakultet — Faculty Laitos — Institution — Department Faculty of Science Department of Physics Tekijä — Författare — Author Julia Martikainen Työn nimi — Arbetets titel — Title Physical modeling for the Vis-SWIR spectrometry of the Chelyabinsk meteorite Oppiaine — Läroämne — Subject Astronomy Työn laji — Arbetets art — Level Aika — Datum — Month and year Sivumäärä — Sidoantal — Number of pages Master’s thesis August 22, 2015 57 pages Tiivistelmä — Referat — Abstract Understanding light scattering on meteorite surfaces is difficult. Multiple factors affect the re- flectance spectra of meteorites, such as space weathering, terrestrial weathering, and shocks. The main focus of this thesis was to investigate how shock induced iron changes meteorite spectra. The reflectance spectra of 30 meteorite pieces were measured with the University of Helsinki spec- trometer in the wavelength range of 300 to 2500 nm. A principal component analysis (PCA) was performed on the spectra and the results were compared with previous studies carried out by Pen- tikäinen et al. (JQSRT, 146, 2014) and Gaffey (NASA PDS, 2001). The analyses show that HED meteorites can be separated from chondrites. However, more HED measurements are needed to verify the validity of the results. The effects of shock induced iron on meteorite spectra were modeled with the SIRIS3 (Muinonen et al., 2009) light-scattering program.