Chemical Composition of Earth,Venus, and Mercury
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

COURT of CLAIMS of THE
REPORTS OF Cases Argued and Determined IN THE COURT of CLAIMS OF THE STATE OF ILLINOIS VOLUME 39 Containing cases in which opinions were filed and orders of dismissal entered, without opinion for: Fiscal Year 1987 - July 1, 1986-June 30, 1987 SPRINGFIELD, ILLINOIS 1988 (Printed by authority of the State of Illinois) (65655--300-7/88) PREFACE The opinions of the Court of Claims reported herein are published by authority of the provisions of Section 18 of the Court of Claims Act, Ill. Rev. Stat. 1987, ch. 37, par. 439.1 et seq. The Court of Claims has exclusive jurisdiction to hear and determine the following matters: (a) all claims against the State of Illinois founded upon any law of the State, or upon an regulation thereunder by an executive or administrative ofgcer or agency, other than claims arising under the Workers’ Compensation Act or the Workers’ Occupational Diseases Act, or claims for certain expenses in civil litigation, (b) all claims against the State founded upon any contract entered into with the State, (c) all claims against the State for time unjustly served in prisons of this State where the persons imprisoned shall receive a pardon from the Governor stating that such pardon is issued on the grounds of innocence of the crime for which they were imprisoned, (d) all claims against the State in cases sounding in tort, (e) all claims for recoupment made by the State against any Claimant, (f) certain claims to compel replacement of a lost or destroyed State warrant, (g) certain claims based on torts by escaped inmates of State institutions, (h) certain representation and indemnification cases, (i) all claims pursuant to the Law Enforcement Officers, Civil Defense Workers, Civil Air Patrol Members, Paramedics and Firemen Compensation Act, (j) all claims pursuant to the Illinois National Guardsman’s and Naval Militiaman’s Compensation Act, and (k) all claims pursuant to the Crime Victims Compensation Act. -
SFSC Search Down to 4
C M Y K www.newssun.com EWS UN NHighlands County’s Hometown-S Newspaper Since 1927 Rivalry rout Deadly wreck in Polk Harris leads Lake 20-year-old woman from Lake Placid to shutout of AP Placid killed in Polk crash SPORTS, B1 PAGE A2 PAGE B14 Friday-Saturday, March 22-23, 2013 www.newssun.com Volume 94/Number 35 | 50 cents Forecast Fire destroys Partly sunny and portable at Fred pleasant High Low Wild Elementary Fire alarms “Myself, Mr. (Wally) 81 62 Cox and other administra- Complete Forecast went off at 2:40 tors were all called about PAGE A14 a.m. Wednesday 3 a.m.,” Waldron said Wednesday morning. Online By SAMANTHA GHOLAR Upon Waldron’s arrival, [email protected] the Sebring Fire SEBRING — Department along with Investigations into a fire DeSoto City Fire early Wednesday morning Department, West Sebring on the Fred Wild Volunteer Fire Department Question: Do you Elementary School cam- and Sebring Police pus are under way. Department were all on think the U.S. govern- The school’s fire alarms the scene. ment would ever News-Sun photo by KATARA SIMMONS Rhoda Ross reads to youngsters Linda Saraniti (from left), Chyanne Carroll and Camdon began going off at approx- State Fire Marshal seize money from pri- Carroll on Wednesday afternoon at the Lake Placid Public Library. Ross was reading from imately 2:40 a.m. and con- investigator Raymond vate bank accounts a children’s book she wrote and illustrated called ‘A Wildflower for all Seasons.’ tinued until about 3 a.m., Miles Davis was on the like is being consid- according to FWE scene for a large part of ered in Cyprus? Principal Laura Waldron. -

Board Certified Fellows
AMERICAN BOARD OF MEDICOLEGAL DEATH INVESTIGATORS Certificant Directory As of September 30, 2021 BOARD CERTIFIED FELLOWS Addison, Krysten Leigh (Inactive) BC2286 Allmon, James L. BC855 Travis County Medical Examiner's Office Sangamon County Coroner's Office 1213 Sabine Street 200 South 9th, Room 203 PO Box 1748 Springfield, IL 62701 Austin, TX 78767 Amini, Navid BC2281 Appleberry, Sherronda BC1721 Olmsted Medical Examiner's Office Adams and Broomfield County Office of the Coroner 200 1st Street Southwest 330 North 19th Avenue Rochester, MN 55905 Brighton, CO 80601 Applegate, MD, David T. BC1829 Archer, Meredith D. BC1036 Union County Coroner's Office Mohave County Medical Examiner 128 South Main Street 1145 Aviation Drive Unit A Marysville, OH 43040 Lake Havasu, AZ 86404 Bailey, Ted E. (Inactive) BC229 Bailey, Sanisha Renee BC1754 Gwinnett County Medical Examiner's Office Virginia Office of the Chief Medical Examiner 320 Hurricane Shoals Road, NE Central District Lawrenceville, GA 30046 400 East Jackson Street Richmond, VA 23219 Balacki, Alexander J BC1513 Banks, Elsie-Kay BC3039 Montgomery County Coroner's Office Maine Office of the Chief Medical Examiner 1430 Dekalb Street 30 Hospital Street PO Box 311 Augusta, ME 04333 Norristown, PA 19404 Bautista, Ian BC2185 Bayer, Lindsey A. BC875 New York City Office of Chief Medical Examiner District 5 and 24 Medical Examiner Office 421 East 26th Street 809 Pine Street New York, NY 10016 Leesburg, FL 34756 Beck, Shari L BC327 Beckham, Phinon Phillips BC2305 Sedgwick Co Reg. Forensic Science Center Virginia Office of the Chief Medical Examiner 1109 N. Minneapolis Northern District Wichita, KS 67214 10850 Pyramid Place, Suite 121 Manassas, VA 20110 Bednar Keefe, Gale M. -

Summary of Sexual Abuse Claims in Chapter 11 Cases of Boy Scouts of America
Summary of Sexual Abuse Claims in Chapter 11 Cases of Boy Scouts of America There are approximately 101,135sexual abuse claims filed. Of those claims, the Tort Claimants’ Committee estimates that there are approximately 83,807 unique claims if the amended and superseded and multiple claims filed on account of the same survivor are removed. The summary of sexual abuse claims below uses the set of 83,807 of claim for purposes of claims summary below.1 The Tort Claimants’ Committee has broken down the sexual abuse claims in various categories for the purpose of disclosing where and when the sexual abuse claims arose and the identity of certain of the parties that are implicated in the alleged sexual abuse. Attached hereto as Exhibit 1 is a chart that shows the sexual abuse claims broken down by the year in which they first arose. Please note that there approximately 10,500 claims did not provide a date for when the sexual abuse occurred. As a result, those claims have not been assigned a year in which the abuse first arose. Attached hereto as Exhibit 2 is a chart that shows the claims broken down by the state or jurisdiction in which they arose. Please note there are approximately 7,186 claims that did not provide a location of abuse. Those claims are reflected by YY or ZZ in the codes used to identify the applicable state or jurisdiction. Those claims have not been assigned a state or other jurisdiction. Attached hereto as Exhibit 3 is a chart that shows the claims broken down by the Local Council implicated in the sexual abuse. -

COURT of CLAIMS of THE
REPORTS ,I OF Cases Argued and Determined IN THE COURT of CLAIMS OF THE STATE OF ILLINOIS . VOLUME 44 Containing cases in which opinions were filed and orders of dismissal entered, without opinion for: Fiscal Year 1992 - July 1, 1991- June 30, 1992 SPRINGFIELD, ILLINOIS 1-993 (Printed by authority of the State of Illinois) (X24064-300-7/93) PREFACE The opinions of the Court of Claims reported herein are published by authority of the provisions of Section 18 of the Court of Claims Act, 705 ILCS 505/1 et seq., formerly 111. Rev. Stat. 1991, ch. 37, par. 439.1 et seq. The Court of Claims has exclusive jurisdiction to hear and determine the following matters: (a) all claims against the State of Illinois founded upon any law of the State, or upon any regulation thereunder by an executive or administrative officer or agency, other than claims arising under the Workers’ Compensation Act or the Workers’ Occupational Diseases Act, or claims for certain expenses in civil litigation, (b) all claims against the State founded upon any contract entered into with the State, (c) all claims against the State for time unjustly served in prisons of this State where the persons imprisoned shall receive a pardon from the Governor stating that such pardon is issued on the grounds of innocence of the crime for which they were imprisoned, (d) all claims against the State in cases sounding in tort, (e) all claims for recoupment made by the State against any Claimant, (f) certain claims to compel replacement of a lost or destroyed State warrant, (g) certain claims based on torts by escaped inmates of State institutions, (h) certain representation and indemnification cases, (i) all claims pursuant to the Law Enforcement Officers, Civil Defense Workers, Civil Air Patrol Members, Paramedics, Firemen & State Employees Compensation Act, (j) all claims pursuant to the Illinois National Guardsman’s Compensation Act, and (k) all claims pursuant to the Crime Victims Compen- sation Act. -
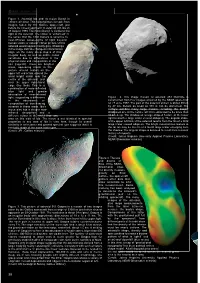
Iso and Asteroids
r bulletin 108 Figure 1. Asteroid Ida and its moon Dactyl in enhanced colour. This colour picture is made from images taken by the Galileo spacecraft just before its closest approach to asteroid 243 Ida on 28 August 1993. The moon Dactyl is visible to the right of the asteroid. The colour is ‘enhanced’ in the sense that the CCD camera is sensitive to near-infrared wavelengths of light beyond human vision; a ‘natural’ colour picture of this asteroid would appear mostly grey. Shadings in the image indicate changes in illumination angle on the many steep slopes of this irregular body, as well as subtle colour variations due to differences in the physical state and composition of the soil (regolith). There are brighter areas, appearing bluish in the picture, around craters on the upper left end of Ida, around the small bright crater near the centre of the asteroid, and near the upper right-hand edge (the limb). This is a combination of more reflected blue light and greater absorption of near-infrared light, suggesting a difference Figure 2. This image mosaic of asteroid 253 Mathilde is in the abundance or constructed from four images acquired by the NEAR spacecraft composition of iron-bearing on 27 June 1997. The part of the asteroid shown is about 59 km minerals in these areas. Ida’s by 47 km. Details as small as 380 m can be discerned. The moon also has a deeper near- surface exhibits many large craters, including the deeply infrared absorption and a shadowed one at the centre, which is estimated to be more than different colour in the violet than any 10 km deep. -

Transneptunian Object Taxonomy 181
Fulchignoni et al.: Transneptunian Object Taxonomy 181 Transneptunian Object Taxonomy Marcello Fulchignoni LESIA, Observatoire de Paris Irina Belskaya Kharkiv National University Maria Antonietta Barucci LESIA, Observatoire de Paris Maria Cristina De Sanctis IASF-INAF, Rome Alain Doressoundiram LESIA, Observatoire de Paris A taxonomic scheme based on multivariate statistics is proposed to distinguish groups of TNOs having the same behavior concerning their BVRIJ colors. As in the case of asteroids, the broadband spectrophotometry provides a first hint about the bulk compositional properties of the TNOs’ surfaces. Principal components (PC) analysis shows that most of the TNOs’ color variability can be accounted for by a single component (i.e., a linear combination of the col- ors): All the studied objects are distributed along a quasicontinuous trend spanning from “gray” (neutral color with respect to those of the Sun) to very “red” (showing a spectacular increase in the reflectance of the I and J bands). A finer structure is superimposed to this trend and four homogeneous “compositional” classes emerge clearly, and independently from the PC analy- sis, if the TNO sample is analyzed with a grouping technique (the G-mode statistics). The first class (designed as BB) contains the objects that are neutral in color with respect to the Sun, while the RR class contains the very red ones. Two intermediate classes are separated by the G mode: the BR and the IR, which are clearly distinguished by the reflectance relative increases in the R and I bands. Some characteristics of the classes are deduced that extend to all the objects of a given class the properties that are common to those members of the class for which more detailed data are available (observed activity, full spectra, albedo). -
The American Nations; Or, Outlines of Their General History, Ancient And
DUMBER 1. SPRIIVO 1836. THE AMERICAN NATIONS; OR, Outlines of A National History; OF THE ANCIENT AND MODERN NATIONS OF NORTH AND SOUTH AMERICA. yx* 4* Of this wide Western Hemisphera^\) Let us retrace the TT history ; * J **/yj ^/ Of all the Nations -L-*-* "* ^ * dwelling herejj^herejk f/* Let us recall the memory ^SSv C\* ^*^^>-^ * O*illf ts ; T^rsss- - ---^ FIRST NUMBER, OR VOMJME: GENERALITIES AND ANNALS. BY PROF. C. S* RAFINESQUE, PHILADEI.PHIA, PUBLISHED BY C. S. RAFINESQUE, NO. 110 NORTH TENTH STREET, SOLD BY THE PRINCIPAL BOOKSELLERS, AND IN LONDON BY O. RICH, IN PARIS BY MEILHAC & BAILLERE. 183G PROSPECTUS. Published quarterly at Five Dollars in advance for Six Numbers or Volumes, simi lar to this, of nearly 300 pages each sep arate Number sold for one Dollar, or more when they will contain maps and illustra tions. A list of Agents will be given hereafter. At present the principal Booksellers may act as such. The Names ot the Subscribers will be printed in a subsequent Number. It is contemplated to conclude these an nals and their illustrations in 12 Numbers or Volumes. Therefore the whole cost to subscribers will only be f 10, for which a complete American Historical Library will be obtained. By remitting $5 to the author, six Vol umes are secured whatever be their future price, and will be sent by mail: a similar sum will be due when the 7th number is issued. Those who may prefer to pay $10 at once, will be deemed Patrons of the work. Whoever subscribes and pays for 5 sets, is entitled to a 6th gratis. -

Walter S. Kiefer
Walter S. Kiefer Lunar and Planetary Institute (281) 486-2110 (Office) 3600 Bay Area Blvd. [email protected] Houston, TX 77058 https://www.lpi.usra.edu/science/staff/kiefer/ Education Ph.D., Planetary Science and Geophysics, California Institute of Technology, 1990 Thesis: Models for the Formation of Highland Regions on Venus M.S., Planetary Science, California Institute of Technology, 1986 B.S., Physics and Astronomy, Texas Christian University, 1984, summa cum laude Honors Thesis: Fourier Transform Infrared Spectroscopy of Clay Minerals and Tar Sands Professional Experience Lunar and Planetary Institute, 1993-present Senior Staff Scientist, 2017-present Manager for Geophysics and Small Bodies Group, 2019-present Staff Scientist, 1997-2016 Research Scientist, 1993-1997 National Research Council Research Associate, Goddard Space Flight Center, 1990-1993 Graduate Research Assistant and Graduate Teaching Assistant, Division of Geological and Planetary Sciences, California Institute of Technology, 1984-1990 Spacecraft Mission and Instrument Development Experience Gravity Recovery and Interior Laboratory (GRAIL) mission Guest Scientist, 2012-2016 DAVINCI+: Deep Atmosphere Venus Investigation of Noble gases, Chemistry, and Imaging+ mission proposal Co-I (NASA Discovery program Phase A Concept Study, 2020-2021) NASA representative on European Space Agency’s Science Study Team for the EnVision mission concept Phase A study (Venus remote sensing orbiter), 2018-2021 NASA Venus Landed Platform Science Working Group, 2018-2020 Seismic Atmospheric -

NAMED VENUSIAN CRATERS; Joel F
NAMED VENUSIAN CRATERS; Joel F. Russell and Gerald G. Schaber, U.S. Geological Survey, 2255 N. Gemini Dr. Flagstaff, AZ 86001 Schaber et al. [I] compiled a database of 841 craters on Venus, based on Magellan coverage of 89% of the planet's surface. That database, derived from coverage of approximately 98% of Venus' surface, has been expanded to 912 craters, ranging in diameter from 1.5 to 280 krn [2]. About 150 of the larger craters were previously identified by Pioneer Venus and Soviet Venera projects and subsequently forrnally named by the International Astronomical Union (IAU). A few of the features identified and nanled as impact craters on Pioneer and Venera images have not been recognized on Magellan images, and therefore the IAU is being requested to drop their names. For example, the feature known as Cleopatra is officially named as a patera, although it is now generally accepted that Cleopatra is a crater [I]. Also, the feature Eve, which has been used to define the prime meridian for Venus, was erroneously identified as an impact feature, but its true morphology has not been determined from Magellan images. The Magellan project has requested the IAU to name hundreds of craters identified by Magellan. At its triennial General Assembly in Buenos Aires in 1991, the IAU [3] gave full approval to names for 102 craters (table 1) in addition to those previously named. At its 1992 meeting, the IAU's Working Group for Planetary System Nomenclature, which screens all planetary names prior to formal consideration by the General Assembly, gave provisional approval to names for an additional 239 Venusian craters. -

Wicked at the Paramount Seattle
DECEMBER 2019 DECEMBER 6 - 15 THE PARAMOUNT THEATRE And to all a good HELP BRING UNSHELTERED FAMILIES INSIDE. Over the last three years, Mary’s Place has moved over 1,400 local unsheltered families into warm, stable homes and provided emergency shelter to hundreds more. Support for their No Child Sleeps Outside campaign from The Starbucks Foundation and the community helps make it all possible. Help reach this year’s $2 million goal by Dec. 31 and bring even more of our neighbors inside, for good. DONATE TO MARY’S PLACE TODAY starbucks.nochildsleepsoutside.org Text FAMILY to 73356 to learn more about how we can help bring unsheltered families inside. Message and data rates may apply. Text STOP to 73356 to opt out, text HELP for more information. By opting in, you agree to receive recurring messages from Starbucks, which may be sent through an automated dialing system. Consent is not required to purchase. © 2019 Starbucks Coffee Company. All rights reserved. December 2019 | Volume 24, No. 2 WELCOMEFrom Seattle Theatre Group, a non-profit arts organization Welcome to the Seattle premiere of THE HARD NUT, the second show of our 2019-2020 Broadway at The Paramount season! Now celebrating its 28th anniversary, Mark Morris Dance Group’s lavish, gender-bent love letter to The Nutcracker is a brilliant blend of ballet, modern, and folk dance that offers an updated classic fairytale that is fun for the entire family. As a season of reflection and thanks is upon us, we want to take this opportunity to thank you for your continued support. -

Exploring Potential Water-Ice Occurrence on Asteroid 4 Vesta Using Orbital Radar Observations by the Dawn Mission
Western Michigan University ScholarWorks at WMU Dissertations Graduate College 12-2018 Exploring Potential Water-Ice Occurrence on Asteroid 4 Vesta Using Orbital Radar Observations by the Dawn Mission Elizabeth M. Palmer Follow this and additional works at: https://scholarworks.wmich.edu/dissertations Part of the Geology Commons Recommended Citation Palmer, Elizabeth M., "Exploring Potential Water-Ice Occurrence on Asteroid 4 Vesta Using Orbital Radar Observations by the Dawn Mission" (2018). Dissertations. 3359. https://scholarworks.wmich.edu/dissertations/3359 This Dissertation-Open Access is brought to you for free and open access by the Graduate College at ScholarWorks at WMU. It has been accepted for inclusion in Dissertations by an authorized administrator of ScholarWorks at WMU. For more information, please contact [email protected]. EXPLORING POTENTIAL WATER-ICE OCCURRENCE ON ASTEROID 4 VESTA USING ORBITAL RADAR OBSERVATIONS BY THE DAWN MISSION by Elizabeth M. Palmer A dissertation submitted to the Graduate College in partial fulfillment of the requirements for the degree of Doctor of Philosophy Geological and Environmental Sciences Western Michigan University December 2018 Doctoral Committee: William A. Sauck, Ph.D., Chair Essam Heggy, Ph.D. Wlodek Kofman, Ph.D. Johnson R. Haas, Ph.D. © 2018 Elizabeth M. Palmer ACKNOWLEDGEMENTS I would like to thank all of my peers who have supported me throughout the many stages of academic and personal growth that have led to the completion of this culminating document. I would also like to thank all those who acted as mentors along the way, especially Essam, who has shared considerable advice on charting the path toward a successful, fulfilling career in the Earth and planetary sciences.