Coastal Ecological Assessment to Support NOAA"S Choptank River Complex Habitat Focus Area: Tred Avon River
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Title 26 Department of the Environment, Subtitle 08 Water
Presented below are water quality standards that are in effect for Clean Water Act purposes. EPA is posting these standards as a convenience to users and has made a reasonable effort to assure their accuracy. Additionally, EPA has made a reasonable effort to identify parts of the standards that are not approved, disapproved, or are otherwise not in effect for Clean Water Act purposes. Title 26 DEPARTMENT OF THE ENVIRONMENT Subtitle 08 WATER POLLUTION Chapters 01-10 2 26.08.01.00 Title 26 DEPARTMENT OF THE ENVIRONMENT Subtitle 08 WATER POLLUTION Chapter 01 General Authority: Environment Article, §§9-313—9-316, 9-319, 9-320, 9-325, 9-327, and 9-328, Annotated Code of Maryland 3 26.08.01.01 .01 Definitions. A. General. (1) The following definitions describe the meaning of terms used in the water quality and water pollution control regulations of the Department of the Environment (COMAR 26.08.01—26.08.04). (2) The terms "discharge", "discharge permit", "disposal system", "effluent limitation", "industrial user", "national pollutant discharge elimination system", "person", "pollutant", "pollution", "publicly owned treatment works", and "waters of this State" are defined in the Environment Article, §§1-101, 9-101, and 9-301, Annotated Code of Maryland. The definitions for these terms are provided below as a convenience, but persons affected by the Department's water quality and water pollution control regulations should be aware that these definitions are subject to amendment by the General Assembly. B. Terms Defined. (1) "Acute toxicity" means the capacity or potential of a substance to cause the onset of deleterious effects in living organisms over a short-term exposure as determined by the Department. -

Nautical Information for Skippers and Crews
Sail Plan Pentagon Sailing Club 2016 Memorial Day Raftup: “STORM FRONT COMING” 2830 May 2016 Nautical Information for Skippers and Crews FLOAT PLAN ******************************************************************************************** References: NOAA Charts 12270 Chesapeake Bay – Chesapeake Eastern Bay and South River; 1:40,000 12266 Chesapeake Bay – Chesapeake – Choptank and Herring Bay; 1:40,000 12280 Chesapeake Bay – 1:200,000 Pentagon Sailing Club RaftUp Guidelines (revised 06/2005; link online at the PSC site under “RaftUp”) Saturday, 28 May 16. Sail from Annapolis, MD the Chesapeake Bay to Trippe Creek, vicinity of Choptank River. Raft up Saturday night (see Navigation below). Distance from Annapolis (direct route past Thomas Point to Choptank River, Tred Avon River, then Trippe Creek and raft up location) is approximately 33 nm Sunday, 29 May 16. Exit Trippe Creek, Tred Avon River, then Choptank River to Campbell’s Boatyard LLC, Bachelor’s Point Marina (Oxford, MD). Dinner will be held at “The Masthead at Pier Street Marina” restaurant in Oxford, MD; cocktails from 5pm, and dinner from 6 to 8pm. Monday, 30 May 16. Sail back to respective points of origin NAVIGATION ******************************************************************************************** Saturday, 28 May: Sail from Annapolis, MD to Raft up destination is in the Trippe Creek vic 038º 42.8 North; 076º 07.3 West. See Chart A and B. From Annapolis R “2” Fl R 2.5s (Lat 038º 56.4 N; Lon 076º 25.3 W) Sail from R “2” Fl R 2.5s 185º M to WP A (Lat 038º -

David Clickner, Et Ux. V. Magothy River Association, Inc., Et Al. No. 13, September Term, 2011, Opinion by Greene, J
David Clickner, et ux. v. Magothy River Association, Inc., et al. No. 13, September Term, 2011, Opinion by Greene, J. REAL PROPERTY LAW – PRESCRIPTIVE EASEMENTS – Public prescriptive rights may be acquired over privately owned portions of beaches located along inland waterways. It was error, however, for the trial court, under the circumstances of this case, to apply the general presumption of adversity to the public use of a beach that was unimproved and in a general state of nature. Instead, the proper presumption was that public use of the land was by permission of the owners. Therefore, the burden was on the claimants to overcome the presumption of permission by proving that the use was, in fact, adverse. IN THE COURT OF APPEALS OF MARYLAND No. 13 September Term, 2011 DAVID CLICKNER, et ux. v. MAGOTHY RIVER ASSOCIATION, INC., et al. Bell, C.J. Harrell Battaglia Greene Adkins Barbera *Murphy, Joseph F., Jr. (Retired, specially assigned), JJ. Opinion by Greene, J. Filed: January 20, 2012 *Murphy, J., participated in the hearing and in the conference of this case in regard to its decision after being recalled pursuant to the Constitution, Art. IV, Sec. 3A but did not participate in the adoption of this opinion. Six individuals and the Magothy River Association, Inc. (collectively, “Association” or “Appellees”) brought suit against the recent purchasers of Dobbins Island, David and Diana Clickner (“Clickners” or “Appellants”), seeking to establish a public right to use a beach located alongside the island’s northern crescent area. Following a bench trial on the merits, the trial judge determined that Appellees had demonstrated the existence of a prescriptive easement on behalf of the public and ordered the removal of portions of a fence erected on the beach by Appellants. -

Maryland Historical Magazine, 1946, Volume 41, Issue No. 4
MHRYMnD CWAQAZIU^j MARYLAND HISTORICAL SOCIETY BALTIMORE DECEMBER • 1946 t. IN 1900 Hutzler Brothers Co. annexed the building at 210 N. Howard Street. Most of the additional space was used for the expansion of existing de- partments, but a new shoe shop was installed on the third floor. It is interesting to note that the shoe department has now returned to its original location ... in a greatly expanded form. HUTZLER BPOTHERSe N\S/Vsc5S8M-lW MARYLAND HISTORICAL MAGAZINE A Quarterly Volume XLI DECEMBER, 1946 Number 4 BALTIMORE AND THE CRISIS OF 1861 Introduction by CHARLES MCHENRY HOWARD » HE following letters, copies of letters, and other documents are from the papers of General Isaac Ridgeway Trimble (b. 1805, d. 1888). They are confined to a brief period of great excitement in Baltimore, viz, after the riot of April 19, 1861, when Federal troops were attacked by the mob while being marched through the City streets, up to May 13th of that year, when General Butler, with a large body of troops occupied Federal Hill, after which Baltimore was substantially under control of the 1 Some months before his death in 1942 the late Charles McHenry Howard (a grandson of Charles Howard, president of the Board of Police in 1861) placed the papers here printed in the Editor's hands for examination, and offered to write an introduction if the Committee on Publications found them acceptable for the Magazine. Owing to the extraordinary events related and the revelation of an episode unknown in Baltimore history, Mr. Howard's proposal was promptly accepted. -

Maryland Stream Waders 10 Year Report
MARYLAND STREAM WADERS TEN YEAR (2000-2009) REPORT October 2012 Maryland Stream Waders Ten Year (2000-2009) Report Prepared for: Maryland Department of Natural Resources Monitoring and Non-tidal Assessment Division 580 Taylor Avenue; C-2 Annapolis, Maryland 21401 1-877-620-8DNR (x8623) [email protected] Prepared by: Daniel Boward1 Sara Weglein1 Erik W. Leppo2 1 Maryland Department of Natural Resources Monitoring and Non-tidal Assessment Division 580 Taylor Avenue; C-2 Annapolis, Maryland 21401 2 Tetra Tech, Inc. Center for Ecological Studies 400 Red Brook Boulevard, Suite 200 Owings Mills, Maryland 21117 October 2012 This page intentionally blank. Foreword This document reports on the firstt en years (2000-2009) of sampling and results for the Maryland Stream Waders (MSW) statewide volunteer stream monitoring program managed by the Maryland Department of Natural Resources’ (DNR) Monitoring and Non-tidal Assessment Division (MANTA). Stream Waders data are intended to supplementt hose collected for the Maryland Biological Stream Survey (MBSS) by DNR and University of Maryland biologists. This report provides an overview oft he Program and summarizes results from the firstt en years of sampling. Acknowledgments We wish to acknowledge, first and foremost, the dedicated volunteers who collected data for this report (Appendix A): Thanks also to the following individuals for helping to make the Program a success. • The DNR Benthic Macroinvertebrate Lab staffof Neal Dziepak, Ellen Friedman, and Kerry Tebbs, for their countless hours in -

Maryland's Lower Choptank River Cultural Resource Inventory
Maryland’s Lower Choptank River Cultural Resource Inventory by Ralph E. Eshelman and Carl W. Scheffel, Jr. “So long as the tides shall ebb and flow in Choptank River.” From Philemon Downes will, Hillsboro, circa 1796 U.S. Geological Survey Quadrangle 7.5 Minute Topographic maps covering the Lower Choptank River (below Caroline County) include: Cambridge (1988), Church Creek (1982), East New Market (1988), Oxford (1988), Preston (1988), Sharp Island (1974R), Tilghman (1988), and Trappe (1988). Introduction The Choptank River is Maryland’s longest river of the Eastern Shore. The Choptank River was ranked as one of four Category One rivers (rivers and related corridors which possess a composite resource value with greater than State signific ance) by the Maryland Rivers Study Wild and Scenic Rivers Program in 1985. It has been stated that “no river in the Chesapeake region has done more to shape the character and society of the Eastern Shore than the Choptank.” It has been called “the noblest watercourse on the Eastern Shore.” Name origin: “Chaptanck” is probably a composition of Algonquian words meaning “it flows back strongly,” referring to the river’s tidal changes1 Geological Change and Flooded Valleys The Choptank River is the largest tributary of the Chesapeake Bay on the eastern shore and is therefore part of the largest estuary in North America. This Bay and all its tributaries were once non-tidal fresh water rivers and streams during the last ice age (15,000 years ago) when sea level was over 300 feet below present. As climate warmed and glaciers melted northward sea level rose, and the Choptank valley and Susquehanna valley became flooded. -
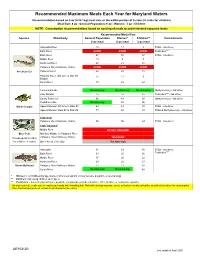
Recommended Maximum Fish Meals Each Year For
Recommended Maximum Meals Each Year for Maryland Waters Recommendation based on 8 oz (0.227 kg) meal size, or the edible portion of 9 crabs (4 crabs for children) Meal Size: 8 oz - General Population; 6 oz - Women; 3 oz - Children NOTE: Consumption recommendations based on spacing of meals to avoid elevated exposure levels Recommended Meals/Year Species Waterbody General PopulationWomen* Children** Contaminants 8 oz meal 6 oz meal 3 oz meal Anacostia River 15 11 8 PCBs - risk driver Back River AVOID AVOID AVOID Pesticides*** Bush River 47 35 27 PCBs - risk driver Middle River 13 9 7 Northeast River 27 21 16 Patapsco River/Baltimore Harbor AVOID AVOID AVOID American Eel Patuxent River 26 20 15 Potomac River (DC Line to MD 301 1511 9 Bridge) South River 37 28 22 Centennial Lake No Advisory No Advisory No Advisory Methylmercury - risk driver Lake Roland 12 12 12 Pesticides*** - risk driver Liberty Reservoir 96 48 48 Methylmercury - risk driver Tuckahoe Lake No Advisory 93 56 Black Crappie Upper Potomac: DC Line to Dam #3 64 49 38 PCBs - risk driver Upper Potomac: Dam #4 to Dam #5 77 58 45 PCBs & Methylmercury - risk driver Crab meat Patapsco River/Baltimore Harbor 96 96 24 PCBs - risk driver Crab "mustard" Middle River DO NOT CONSUME Blue Crab Mid Bay: Middle to Patapsco River (1 meal equals 9 crabs) Patapsco River/Baltimore Harbor "MUSTARD" (for children: 4 crabs ) Other Areas of the Bay Eat Sparingly Anacostia 51 39 30 PCBs - risk driver Back River 33 25 20 Pesticides*** Middle River 37 28 22 Northeast River 29 22 17 Brown Bullhead Patapsco River/Baltimore Harbor 17 13 10 South River No Advisory No Advisory 88 * Women = of childbearing age (women who are pregnant or may become pregnant, or are nursing) ** Children = all young children up to age 6 *** Pesticides = banned organochlorine pesticide compounds (include chlordane, DDT, dieldrin, or heptachlor epoxide) As a general rule, make sure to wash your hands after handling fish. -

Bicycle Map Bicycle
BicBiyccleyc Male Map p BicBicycley cleSaf Setyafety MarylandMaryland law requires law requires all bicyclists all bicyclists under under the age the of age 16 ofto 16wear to weara bicycle a bicycle safetysafety helmet helmet when when riding riding on public on public propert property. Thisy. includesThis includes roadways, roadways, trails trailsand sidewalks. and sidewalks. Some Some local localjurisdictions jurisdictions maintain maintain their owntheir localown local rules:rules: • Ride• Rideon the on right the rightside of side the of road the withroad thewith tra theffic tra flofficw, flonotw against, not against it; it; • Obey• Obey traffic tra signsffic signsand signals; and signals; • Never• Never change change directions directions or lanes or laneswithout without first looking first looking behind behind you, andyou, and alwaysalways use the use correct the correct hand handsignals. signals. Use your Use leftyour arm left for arm all for hand all handsignals: signals: Left turn:Left turn: After After checking checking behind behind you, holdyou, yourhold armyour arm straightstraight out to out the to left the and left ride and forward ride forward slowl slowly. y. Stop:S Aftertop: After checking checking behind behind you, bendyou, bendyour elboyourw elbo, point-w, point- ing youring armyour downward arm downward in an inupside an upside down down “L” shape “L” shape and and comecome to a stop. to a stop. RightRight turn: turn: After After checking checking behind behind you, bendyou, bendyour elboyourw elbo, w, holdingholding your armyour up arm in upan in“L” an shape, “L” shape, and ride and forward ride forward slowlslowly. Or,y hold. Or, yourhold rightyour rightarm straight arm straight out from out fromyour side.your side. -

Title 08 DEPARTMENT of NATURAL RESOURCES
The Secretary of the Maryland Department of Natural Resources proposes to amend/add Regulation .01 - .03 under COMAR 08.18.27 The purpose of this action is to update and clarify existing boating laws and coordinates of certain boundaries. This will ensure safe boating on Maryland’s waterways. Items in [parenthesis] are being deleted; items in italics are being added. Title 08 DEPARTMENT OF NATURAL RESOURCES Subtitle 18 BOATING — SPEED LIMITS AND OPERATION OF VESSELS Chapter 27 Tred Avon River Authority: Natural Resources Article, §§8-703 and 8-704, Annotated Code of Maryland .01 Tred Avon River. The Tred Avon River encompasses all the waters of the Tred Avon River and its tributaries northeasterly of a line [from Lat. 38°40'15.09”N., Long. 76°11'51.62”W., running 073° True to the opposite shore, Lat. 38°40'32.92”N., Long. 76°10'37.21”W.] beginning at a point, at or near Lat. 38° 40.250' N., Long. 76° 11.867' W., then running 73° (True) to a point, at or near Lat. 38° 40.550' N., Long. 76° 10.617' W. .02 Tred Avon River at Oxford. The Tred Avon River at Oxford encompasses the area beginning at a point,[Lat. 38°41'35.5"N., Long. 76°10'36.5"W., a line running 279° True to Lat. 38°41'36.4"N., Long. 76°10'43.4"W., then running 64° True to USCG DBN 4 at Lat. 38°41'52.1"N., Long. 76°10'3.2"W., then 178° True to the shore at the east side of Town Creek, Lat. -

DESTINATION GUIDE Contents Easton 2, 17-22 Bellevue 16 St
DESTINATION GUIDE Contents Easton 2, 17-22 Bellevue 16 St. Michaels 4, 27-31 Bozman 16 Oxford 6, 24 & 25 Claiborne 16 Tilghman Island 8, 32-34 Cordova 16 Trappe 10, 35 Royal Oak 26 Wye Mills 11, 35 Sherwood 26 Green Talbot 12 Wittman 35 On the Water 13 Calendar of Events 36 On the Table 14 Talbot County Office of Tourism • 11 S. Harrison Street • Easton, MD 21601 410-770-8000 www.tourtalbot.org Written by Kessler Burnett Design by Eclectic Graphics, Easton, MD www.eclecticgraphics.com Photo Credits: Linda Farwell Photography, Richard Dorbin www.paragonlight.com, HARB_1059_Talbot county visitor 2/14/08 3:19 PM Page 1 Kirsten Beckerman, Dave Harp/ChesapeakePhotos.com, Bill Thompson, and Scott Suchman/Photographer. Harbourtowne, St. Michaels, MD • 111 Waterfront Guest Rooms • Weddings • Waterfront Suites with Wood Burning Stoves • Massage Services • 18-hole Pete Dye Championship Golf Course • Waterfront Dining • Pool/Tennis Courts • Meeting Facilities Romantic and Golf Getaway Packages Available Call for Additional Information 800.446.9066 | harbourtowne.com 1 We’re teeming with incredibly talented chefs who spoil their food- loving locals and guests with some of the most inventive menus on the East Coast, so say restaurant critics from the likes of Travel + Leisure and The New York Times. From Thai to Tidewater delicacies, Sushi to steakhouse staples, we have a memorable fix for every craving. Feel the need for (moderate) speed? Several of our cozy inns treat their guests to bikes, a playful way to explore our charming setting. Pedal past the Historical Society of Talbot County’s lush Federal gardens, down our residential streets canopied by rows of towering oaks, or take a spin through town and break for a frothy cappuccino. -

Watersheds.Pdf
Watershed Code Watershed Name 02130705 Aberdeen Proving Ground 02140205 Anacostia River 02140502 Antietam Creek 02130102 Assawoman Bay 02130703 Atkisson Reservoir 02130101 Atlantic Ocean 02130604 Back Creek 02130901 Back River 02130903 Baltimore Harbor 02130207 Big Annemessex River 02130606 Big Elk Creek 02130803 Bird River 02130902 Bodkin Creek 02130602 Bohemia River 02140104 Breton Bay 02131108 Brighton Dam 02120205 Broad Creek 02130701 Bush River 02130704 Bynum Run 02140207 Cabin John Creek 05020204 Casselman River 02140305 Catoctin Creek 02130106 Chincoteague Bay 02130607 Christina River 02050301 Conewago Creek 02140504 Conococheague Creek 02120204 Conowingo Dam Susq R 02130507 Corsica River 05020203 Deep Creek Lake 02120202 Deer Creek 02130204 Dividing Creek 02140304 Double Pipe Creek 02130501 Eastern Bay 02141002 Evitts Creek 02140511 Fifteen Mile Creek 02130307 Fishing Bay 02130609 Furnace Bay 02141004 Georges Creek 02140107 Gilbert Swamp 02130801 Gunpowder River 02130905 Gwynns Falls 02130401 Honga River 02130103 Isle of Wight Bay 02130904 Jones Falls 02130511 Kent Island Bay 02130504 Kent Narrows 02120201 L Susquehanna River 02130506 Langford Creek 02130907 Liberty Reservoir 02140506 Licking Creek 02130402 Little Choptank 02140505 Little Conococheague 02130605 Little Elk Creek 02130804 Little Gunpowder Falls 02131105 Little Patuxent River 02140509 Little Tonoloway Creek 05020202 Little Youghiogheny R 02130805 Loch Raven Reservoir 02139998 Lower Chesapeake Bay 02130505 Lower Chester River 02130403 Lower Choptank 02130601 Lower -

Non-Tidal Patuxent River Lower and Middle Watersheds Sediment TMDL Restoration Plan June 2019
NON TIDAL PATUXENT RIVER LOWER AND MIDDLE WATERSHEDS SEDIMENT TMDL RESTORATION PLAN JUNE 2019 DRAFT FOR PUBLIC COMMENT Draft Non-Tidal Patuxent River Lower and Middle Watersheds Sediment TMDL Restoration Plan June 2019 NON TIDAL PATUXENT RIVER LOWER AND MIDDLE WATERSHEDS SEDIMENT TMDL RESTORATION PLAN JUNE 2019 DRAFT FOR PUBLIC COMMENT PREPARED FOR Anne Arundel County Department of Public Works Watershed Protection and Restoration Program 2662 Riva Road, P.O. Box 6675 Annapolis, Maryland 21401 PREPARED BY LimnoTech 1015 18th Street NW Suite 900 Washington, DC 20003 Anne Arundel County DPW ii Draft Non-Tidal Patuxent River Lower and Middle Watersheds Sediment TMDL Restoration Plan June 2019 TABLE OF CONTENTS 1 Introduction ....................................................................................................................................1 1.1 Background and Purpose ........................................................................................................................ 1 1.2 TMDL Allocated and Planned Loads Summary ....................................................................................... 2 1.3 Restoration Plan Elements and Structure ............................................................................................... 3 2 Watershed Characteristics ...............................................................................................................6 2.1 Lower Patuxent ......................................................................................................................................