Trace Gas Spectrometry Using Quantum Cascade Lasers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Warminster 2017 Vehicles & Running Lines
Warminster Vintage Bus Running Day - 1st October 2017 Vehicles expected to be in use updated on 30th September 2017 Registration Vehicle Seating New New to AHU 803 Bristol JO5G / Bristol B35R 1934 Bristol Tramways JDV 754 Bedford OB / Duple Vista C23F 1947 Woolacombe & Mortehoe Coaches JNN 384 Leyland Titan PD1 / Duple L29/26F 1947 Barton Transport LHT 911 Bristol L5G / BBW B35R 1948 Bristol Omnibus ► JLJ 403 Leyland Tiger PS2 / Burlingham C35F 1949 Bournemouth Transport KLJ 749 Bristol LL6G / Portsmouth Aviation B36R 1950 Hants & Dorset LLU 957 Leyland PD2 / Leyland H30/26R 1950 London Transport MXX 398 AEC Regal IV / MCW B41F 1953 London Transport MOD 973 Bristol LS6G / ECW C39F 1959 Royal Blue (Southern National) X STP 995 Leyland PD2 / Metro-Cammell H36/28R 1959 Portsmouth Corporation ► 969 EHW Bristol LD6G ECW H33/25R 1959 Bath Tramways / Bristol Omnibus WCG 104 Leyland Tiger Cub / Weymann B45F 1959 King Alfred Motor Services ► OVL 494 Bristol SC4LK / ECW B35F 1960 Lincolnshire Road Car 56 GUO Bristol MW / ECW C39F 1961 Royal Blue (Westen National) 1013 MW Leyland Atlantean PDR1 / Weymann L39/34F 1962 Silver Star Motor Services 270 KTA Bristol SUL4A / ECW DP33F 1962 Western National 5 CLT AEC Routemaster / Park Royal H34/30R 1962 London Transport KPM 91E Bristol FLF6G / ECW O38/32F 1967 Brighton Hove & District KED 546F Leyland Panther Cub / East Lancs B41F 1968 Warrington Corporation X OAX 9F Bristol RELH6L / ECW C41F 1968 Red & White Services THU 354G Bristol RESL6L / ECW B43F 1969 Bristol Omnibus ► AFM 103G Bristol RELH6G / ECW -

Cardiff Corporation Transport City of Cardiff Transport Cardiff Bus
Cardiff Corporation Transport City of Cardiff Transport Cardiff Bus Details of vehicles purchased new and secondhand from 1947 onwards Part 5 – 1981 to 1986 Delivery of the bus-seated East Lancs-bodied Leyland Olympians continued from 1983 into 1984, the last of the batch, 519 (A519 VKG) is seen in Cardiff Bus Station on 12th July 1988. The last two deliveries of Olympians would feature coach seating. All photographs by Mike Street Cardiff’s Buses Page 1 1981 The first East Lancs-bodied Leyland Olympian was 501 (LBO 501X), new in November 1981. It is seen at Sloper Road depot in March 1983. 501 LBO 501X Leyland ONLXB/1R East Lancs H43/31F Notes Following the 1978 vehicle evaluation mentioned in Part 4, 36 East Lancs bodied Leyland Olympians and 36 Northern Counties-bodied Ailsa B55-10s were ordered. These entered service between 1981 and 1986. 501 (LBO 501X) had a narrow white band above the lower deck windows, forcing the fleet name and crest to be relocated to the lower deck panels. Year of withdrawal 1998: 501 Cardiff’s Buses Page 2 1982 Delivery of the Northern Counties-bodied Ailsa AB55-10s proceeded at a quicker pace than the Olympians. The first, 401 (NDW 401X), was handed over at the City Hall in March 1982 and eighteen had been delivered by the end of that month. 401 – 418 NDW 401 – 418X Ailsa B55-10 Northern Counties H39/35F Notes 401 – 418 (NDW 401 – 418X) In 1998/9 a number of these vehicles were refurbished, fitted with electronic destination screens and repainted in a modified livery. -

John Fishwick & Sons 1907-2015
John Fishwick & Sons 1907-2015 Contents John Fishwick & Sons - Fleet History 1907 - 2015 Page 3 John Fishwick & Sons - Bus Fleet List 1907 - 2015 Page 8 Cover Illustration: Preserved 1958 Leyland PD2/40 with Weymann lowbridge 58-seat bodywork. (LTHL collection). First Published 2018. 2nd edition May 2020. With thanks to Roy Marshall, RHG Simpson, Joe Gornall (courtesy Malcolm Jones), Frans Angevaare and Alan Sansbury for illustrations. © The Local Transport History Library 2018. (www.lthlibrary.org.uk) For personal use only. No part of this publication may be reproduced, stored in a retrieval system, transmitted or distributed in any form or by any means, electronic, mechanical or otherwise without the express written permission of the publisher. In all cases this notice must remain intact. All rights reserved. PDF-119-2 Page 2 John Fishwick & Sons 1907-2015 After a spell working with the Leyland Steam Motor Company in Leyland, John Fishwick decided to start his own haulage business. In 1907 he purchased a steam wagon from his former employers and began hauling rubber from the local works to Liverpool and Manchester. In 1910 he purchased another Leyland vehicle - this time a Leyland X-type with petrol engine that was used as a lorry but could be fitted with a very basic style of wagonette body seating 30 passengers for a Saturday only service to Leyland market from Eccleston, that commenced in 1911. More vehicles followed, most of which had interchangeable bodies for use as a lorry as well as a bus. Soon John Fishwick was operating a number of routes serving Preston, Chorley and Ormskirk. -

Buses That We Don't Have Current Details For
Check List - buses that we don't have current details for The main lists on our website show the details of the many thousands of open top buses that currently exist throughout the world, and those that are listed as either scrapped or for scrap. However, there are a number of buses in our database that we don’t have current details for, that could still exist or have been scrapped. The buses listed on this page are those that we need to confirm the location and status of. These buses do not appear on any of our other lists, so if you're looking for a particular vehicle, it could be here. Please have a look at this page and if you can update any of it, even if only a small piece of information that helps to determine where a bus is now, then please contact us using the link button on the Front Page. The buses are divided into lists in Chassis manufacturer order. ? REG NO / LICENCE PLATE CHASSIS BODY STATUS/LAST KNOWN OWNER J2374 ? ? Last reported with JMT in 1960s, no further trace AEC Regent REG NO / LICENCE PLATE CHASSIS BODY STATUS/LAST KNOWN OWNER AUO 90 AEC Regent Unidentified Devon General AUO 91 AEC Regent Unidentified Devon General GW 6276 AEC Regent Brighton & Hove Acquired by Southern Vectis (903) from Brighton Hove and District in 1955. Sold, 1960, not traced further. GW 6277 AEC Regent Brighton & Hove Acquired by Southern Vectis (902) from Brighton Hove and District in 1955, never entered service, disposed of in 1957. -
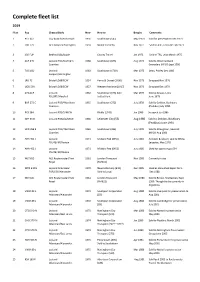
Complete Fleet List
Complete fleet list 2019 Fleet Reg Chassis/Body New New to Bought Comments 1 PUF 647 Guy Arab IV/Park Royal 1956 Southdown (547) May 1970 Sold for preservation Dec 1973 2 LDB 779 AEC Reliance Harrington 1959 Nesbit Somerby Nov 1971 Sold to ABC, Leicester Feb 1973 3 456 FUP Bedford SB1/Duple County Travel Jan 1973 Sold to TRS, Leics March 1975 4 XUF 845 Leyland PD3/Northern 1958 Southdown (845) Aug 1973 Sold to West Auckland Counties Grenaders (NPSV) Sept 1980 5 749 DCD Leyland 1963 Southdown (1749) Mar 1975 Dean, Paisley Dec 1985 Leopard/Harrington 6 LRU 72 Bristol LD6B/ECW 1954 Hants & Dorset (1406) Nov 1975 Scrapped Nov 1976 7 UOD 500 Bristol LD6B/ECW 1957 Western National (1917) Nov 1976 Scrapped Dec 1979 8 273 AUF Leyland 1962 Southdown (673) Sold Mar 1977 Sold to Straws, Leics PSU3RT/Marshall to East Kent June 1978 9 BUF 272 C Leyland PD3/4Northern 1965 Southdown (272) July 1978 Sold to Seddon, Bushbury Counties (Peakbus) July 1993 10 PCK 384 Leyland PD3/5/MCW Ribble (1743) Jan 1986 Scrapped Jan 1986 11 GRY 55 D Leyland PD3A/2/MCW 1966 Leicester City (55) Aug 1980 Sold to Seddon, Bushbury (Peakbus) June 1994 12 HCD 356 E Leyland PD3/4Northern 1965 Southdown (356) July 1978 Sold to Strangman, Gawcott Counties (NPSV) Aug 1981 13 AHA 451 J Leyland 1971 Midland Red (6451) July 1983 Accident & rebuilt, sold to White, PSU4B/4R/Plaxton Leicester, May 1978 14 AHA 452 J Leyland 1971 Midland Red (6452) July 1985 Used for spares Aug 1994 PSU4B/4R/Plaxton 15 WLT 655 AEC Routemaster/Park 1961 London Transport Nov 1985 Currently in use Royal (RM655) 16 WTN 640 H Leyland Atlanteean 1970 Newcastle Corp (640) Dec 1985 Used as store shed depot form PDR2/3R/Alexander Sold to Lloyd Dec 1988 17 WLT 621 AEC Routemaster/Park 1961 London Transport May 1986 Sold to Brown, Shaftesbury Sept Royal (RM621) 1990. -

Fleet Lists - Pennsylvania, USA
Fleet Lists - Pennsylvania, USA This is our list of current open top buses in Pennsylvania, USA MEDIA - Double Decker Pizza The status/use of this bus is unknown to us. If you can supply any more information, please contact us. Fleet List FLEET NO REG NO CHASSIS / BODY LAYOUT LIVERY PREVIOUS KNOWN OWNER - BA 73487 MCW Metrobus Mk.II / MCW O??/??F red ? , by 4/18; LKO: The Big Red Bus Company Inc., Hollywood - gone by 7/16 Previous Registration Numbers BA 73487 was previously 7DIV143, B776 AOC PHILADELPHIA - 76 Carriage Co. Inc. {Big Bus Philadelphia} Buses used on sightseeing tours. (Big Bus Company franchise) Fleet List FLEET NO REG NO CHASSIS / BODY LAYOUT LIVERY PREVIOUS KNOWN OWNER 305 no plate yet Gillig / ? O??/??F ? ?, by 12/19 310 BA 56775 (w) Bristol VRT / ECW O--/27F+ BIG BUS TOUR HOP ON HOP OFF promotional livery (withdrawn awaiting sale) Blackstone Valley Tourism Council, Pawtucket, Rhode Island, New England, 6/05 (Used as a static ticket bus.) 315 no plate yet Gillig / ? O??/??F ? ?, by 12/19 320 BA 56780 (w) Bristol VRT / ECW O??/??F+ allover promotional pictorial vinyl (withdrawn, awaiting sale) Thomas Jefferson University, Philadelphia, by 12/06 325 BA 71613 Gillig / ? O??/??F advert for 'Eastern Street Penitentiary' ?, by 7/16 330 BA 56781 (w) Bristol VRT / ECW O43/27F+ BIG BUS TOURS PHILLY BY NIGHT promotional livery (last active Bristol VR Autobus Galland Ltd. (702), Laval, Quebec, Canada, used) 5/05 335 BA 71699 Gillig / ? O??/??F ? ?, by 12/19 340 BA 56779 (w) Bristol VRT / ECW O43/27F+ advert for 'Rum Chata' (withdrawn -

Buses Scrapped, Or Sold for Scrap AEC Regent II
Buses scrapped, or sold for scrap This is our list of buses that have been scrapped, sold for scrap, or are thought to no longer exist. These buses can be considered to fall into two categories. Buses scrapped In most cases, these buses will have been sold to a vehicle dismantler or scrap dealer, where they would be stripped of any useful parts and the remains cut up, so that the vehicle no longer exists. In some cases the dismantling or cutting up has been done by the bus owner and the remains sold to a scrap dealer. Buses sold for scrap When buses are sold for scrap, it is probably the intention of the seller that the vehicle should be dismantled. Many of our records are conclusive, in that we record that the buses no longer exist. However, other buses are shown as ‘scrapped’, but it is possible that while we believe they were sold for dismantling, the physical destruction process may not yet have happened, and the buses may still exist on the dealer’s premises in either complete form or in a delapitated condition. We will update the records on this list in due course to identify any buses that we thought had been destroyed that perhaps do still exist in ‘sold for scrap’ condition. The buses are grouped into ‘Chassis Make’ lists, in registration/licence plate order. As always, if you can update or correct anything on this list, please contact us. Please note that there may be some conflicts with buses listed on the ‘To Check’ page, where this page has been updated and the To Check page has still to be updated. -

Devon General Centenary Bus Rally and Running Day List of Entries As at 29Th July 2019
DEVON GENERAL CENTENARY BUS RALLY AND RUNNING DAY LIST OF ENTRIES AS AT 29TH JULY 2019 Entry No. Year Reg. No. Fleet No. Chassis Body Seating Original operator Scheduled in service Vehicles owned by Devon General & successors D13 1934 OD 7497 DR210 AEC Regent I Short Bros. O31/24R Devon General S5 1946 HTT 487 SR487 AEC Regal Weymann B35F Devon General Yes D10 1949 KOD 585 DR585 AEC Regent III Weymann H30/26R Devon General D2 1951 MTT 640 DL640 Leyland Titan PD2 Leyland L27/26R Devon General Yes D14 1953 NTT 679 DR679 AEC Regent III Weymann H30/26R Devon General Yes D1 1953 ETT 995 DR705 AEC Light Six Saunders-Roe H30/26R Devon General D4 1956 ROD 765 DR765 AEC Regent V Metro-Cammell H33/26RD Devon General D33 1956 LRV 992 992 Leyland Titan PD2 Metro-Cammell O33/26R Portsmouth City Transport Yes S1 1957 VDV 798 SR798 AEC Reliance Weymann B41F Devon General D5 1957 VDV 817 DR817 AEC Regent V Metro-Cammell H33/26R Devon General Yes D37 1957 VDV 818 DR818 AEC Regent V Metro-Cammell O33/26R Devon General S7 1958 XTA 839 SN839 Albion Nimbus Willowbrook B31F Devon General D17 1959 872 ATA DL872 Leyland Atlantean Metro-Cammell H44/32F Devon General Yes S20 1959 890 ADV TCR890 AEC Reliance Willowbrook C41F Devon General D6 1961 MSJ 499 DL925 Leyland Atlantean Metro-Cammell O44/31F Devon General Yes D36 1961 928 GTA 928 Leyland Atlantean Metro-Cammell O44/31F Devon General D11 1961 931 GTA 931 Leyland Atlantean Metro-Cammell O44/31F Devon General Yes S2 1962 960 HTT TCR960 AEC Reliance Willowbrook C41F Devon General S8 1962 270 KTA 420 Bristol -

Atlanteans in the South and West the Impact of ATLANTEANS in the South and West
a Impact of Atlanteans in the South and West Atlanteans in the South and Impact of The impact of ATLANTEANS in the South and West David Toy David Toy, a former Chief Engineer and transport enthusiast now enjoying retirement, describes how the introduction of the rear-engined Leyland Atlantean impacted on the areas in which he was working – the south and west of England. Fully illustrated with sections on the competition it provides a fascinating review of a slice of history which lasted for 40 years. 128 PIKES LANE GLOSSOP DERBYSHIRE SK13 8EH (01457 861508 E-MAIL [email protected] INTERNET www.venturepublications.co.uk ISBN 978 1905 304 25 7 David Toy This free edition is provided by MDS Book Sales during the coronavirus lockdown. There’s no charge and it may be distributed as you wish. If you’d like to make a donation to our charity of choice - The Christie, Europe’s largest specialist cancer centre - there’s a link here. The impact of ATLANTEANS in the South and West David Toy © 2011 Venture Publications Ltd ISBN 978 1905 304 34 9 All rights reserved. Except for normal review purposes no part of this book maybe reproduced or utilised in any form by any means, electrical or mechanical, including photocopying, recording or by an information storage and retrieval system, without the prior written consent of Venture Publications Ltd, Glossop, Derbyshire, SK13 8EH. The only single-deck Atlanteans supplied to an operator in the South and West were twelve delivered to Portsmouth, with Seddon bodies as seen below. -

Fleet Lists - Gloucestershire, England
Fleet Lists - Gloucestershire, England This is our list of current open top buses in Gloucestershire, England BOURTON-ON-THE-HILL - Troopers Lodge Garage Limited (Troopers Lodge Motor Services) Bus used for special events/private hire. Fleet List FLEET NO REG NO CHASSIS / BODY LAYOUT LIVERY PREVIOUS KNOWN OWNER(S) 84 DHC 784E Leyland Titan PD2 / East Lancs O32/28R TROOPERS LODGE MOTOR SERVICES (dark blue & primrose) Eastwood (Private), Bourton-on-the-Hill, 4/21; David Mulpeter / Seaford and District Motor Services Limited, Ringmer, East Sussex, 12/20; Southdown Historic Vehicles Ltd., Worthing, 10/13 BRISTOL - Abus Ltd. Buses fitted with convertible open tops, but currently normally used on local bus services with roofs fitted. Fleet List FLEET NO REG NO CHASSIS / BODY LAYOUT LIVERY PREVIOUS KNOWN OWNER(S) - M645 RCP DAF DB250 / Northern Counties CO47/30F www.abus.co.uk (cream & maroon) Go South Coast Events Fleet, 12/12 Palatine II BRISTOL - Bath Bus Company Ltd. (Tootbus) [RATP Dev / Extrapolitan Sightseeing Group] Buses used on sightseeing tours. Fleet List FLEET NO REG NO CHASSIS / BODY LAYOUT LIVERY PREVIOUS KNOWN OWNER(S) 360 AE60 GRU Volvo B9TL / MCV DD103 O49/31F red with large white Tudor rose Transferred from Bath, 7/21 & from Windsor, by 9/17; Ensign, dealer, Purfleet, 1/12 703 WX15 OFT Volvo B5TL / UNVI Urbis 2.5 DD PO53/23D TO-OTBUS BRISTOL (red with large white Tudor rose) transferred from Bath, 5/21; new as part open top, 8/15 704 WX15 OFU Volvo B5TL / UNVI Urbis 2.5 DD PO53/23D TO-OTBUS BRISTOL (red with large white Tudor rose) transferred from Bath, 5/21; new as part open top, 8/15 BRISTOL - Bristol Vintage Bus Group Buses preserved/restored. -

Gordon Mills, Trust Chairman Chairman's
Chairman's Update …. It is this time of year, when we are making our return to The Scottish Charities Regulator, that we reflect on the last season and what we have achieved with restoration and repairs. It was a busy year for events, and we have set out on major projects in the workshops. The events season was dominated by the display at Castlegate and the Open Day at Alford which, thankfully was dry again and both these were a big success with numbers up from last time. We were asked to do three days at Balmoral during 2019, carrying visitors from the gate to the visitor centre, these days are thoroughly enjoyable and help to keep the wheels turning, all were completed without any problems. We were helped this year by Graham Davidson who is one of the rare breed of “crash gearbox” drivers and is now familiar with PA171. The “Blast from the past” event at Thainstone Mart was attended by Leyland National 74 and received much attention being the only bus there. This show is well attended with a tremendous atmosphere and well worth a visit. The BA Stores Open Weekend and ploughing competition was plagued by rain however, taking a bus to these venues always provides a dry zone where the public can come out of the wet and see what we get up to,131 was our entrant this year. The workshops have been very busy with Daimler CVD6 number 11 in for full restoration and much progress has already been achieved. Daimler 14 similar to 11 but with some of its original Walker bodywork has gone to Lathalmond where it also is under- going a full restoration into its “as delivered” layout. -
Warminster 2017 Vehicles & Running Lines
Warminster Vintage Bus Running Day - 1st October 2017 Vehicles expected to be in use as at September 2017 Registration Vehicle Seating New New to AHU 803 Bristol JO5G / Bristol B35R 1934 Bristol Tramways JDV 754 Bedford OB / Duple Vista C23F 1947 Woolacombe & Mortehoe Coaches JNN 384 Leyland Titan PD1 / Duple L29/26F 1947 Barton Transport LHT 911 Bristol L5G / BBW B35R 1948 Bristol Omnibus KLJ 749 Bristol LL6G / Portsmouth Aviation B36R 1950 Hants & Dorset LLU 957 Leyland PD2 / Leyland H30/26R 1950 London Transport MXX 398 AEC Regal IV / MCW B41F 1953 London Transport MOD 973 Bristol LS6G / ECW C39F 1959 Royal Blue (Southern National) STP 995 Leyland PD2 / Metro-Cammell H36/28R 1959 Portsmouth Corporation WCG 104 Leyland Tiger Cub / Weymann B45F 1959 King Alfred Motor Services 56 GUO Bristol MW / ECW C39F 1961 Royal Blue (Westen National) 1013 MW Leyland Atlantean PDR1 / Weymann L39/34F 1962 Silver Star Motor Services 270 KTA Bristol SUL4A / ECW DP33F 1962 Western National 5 CLT AEC Routemaster / Park Royal H34/30R 1962 London Transport KPM 91E Bristol FLF6G / ECW O38/32F 1967 Brighton Hove & District KED 546F Leyland Panther Cub / East Lancs B41F 1968 Warrington Corporation OAX 9F Bristol RELH6L / ECW C41F 1968 Red & White Services THU 354G Bristol RESL6L / ECW B43F 1969 Bristol Omnibus RDV 423H Bristol RELH6G / ECW C45F 1970 Western National (Royal Blue) # TRU 947J Bristol RELL6G / ECW DP50F 1970 Wilts & Dorset YHT 802J Bristol RESL6L / ECW B43F 1970 Bristol Omnibus DAE 511K Bristol RELL6L / ECW B50F 1971 Bristol Omnibus GLJ 681N Leyland