05 Vasc Pl II-Ferns.Pptx
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gymnosperms the MESOZOIC: ERA of GYMNOSPERM DOMINANCE
Chapter 24 Gymnosperms THE MESOZOIC: ERA OF GYMNOSPERM DOMINANCE THE VASCULAR SYSTEM OF GYMNOSPERMS CYCADS GINKGO CONIFERS Pinaceae Include the Pines, Firs, and Spruces Cupressaceae Include the Junipers, Cypresses, and Redwoods Taxaceae Include the Yews, but Plum Yews Belong to Cephalotaxaceae Podocarpaceae and Araucariaceae Are Largely Southern Hemisphere Conifers THE LIFE CYCLE OF PINUS, A REPRESENTATIVE GYMNOSPERM Pollen and Ovules Are Produced in Different Kinds of Structures Pollination Replaces the Need for Free Water Fertilization Leads to Seed Formation GNETOPHYTES GYMNOSPERMS: SEEDS, POLLEN, AND WOOD THE ECOLOGICAL AND ECONOMIC IMPORTANCE OF GYMNOSPERMS The Origin of Seeds, Pollen, and Wood Seeds and Pollen Are Key Reproductive SUMMARY Innovations for Life on Land Seed Plants Have Distinctive Vegetative PLANTS, PEOPLE, AND THE Features ENVIRONMENT: The California Coast Relationships among Gymnosperms Redwood Forest 1 KEY CONCEPTS 1. The evolution of seeds, pollen, and wood freed plants from the need for water during reproduction, allowed for more effective dispersal of sperm, increased parental investment in the next generation and allowed for greater size and strength. 2. Seed plants originated in the Devonian period from a group called the progymnosperms, which possessed wood and heterospory, but reproduced by releasing spores. Currently, five lineages of seed plants survive--the flowering plants plus four groups of gymnosperms: cycads, Ginkgo, conifers, and gnetophytes. Conifers are the best known and most economically important group, including pines, firs, spruces, hemlocks, redwoods, cedars, cypress, yews, and several Southern Hemisphere genera. 3. The pine life cycle is heterosporous. Pollen strobili are small and seasonal. Each sporophyll has two microsporangia, in which microspores are formed and divide into immature male gametophytes while still retained in the microsporangia. -

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

Genetic Differentiation and Polyploid Formation Within the Cryptogramma Crispa Complex (Polypodiales: Pteridaceae)
Turkish Journal of Botany Turk J Bot (2016) 40: 231-240 http://journals.tubitak.gov.tr/botany/ © TÜBİTAK Research Article doi:10.3906/bot-1501-54 Genetic differentiation and polyploid formation within the Cryptogramma crispa complex (Polypodiales: Pteridaceae) Jordan METZGAR*, Mackenzie STAMEY, Stefanie ICKERT-BOND Herbarium (ALA), University of Alaska Museum of the North and Department of Biology and Wildlife, University of Alaska Fairbanks, Fairbanks, AK, USA Received: 28.01.2015 Accepted/Published Online: 14.07.2015 Final Version: 08.04.2016 Abstract: The tetraploid fern Cryptogramma crispa (L.) R.Br. ex Hook. is distributed across alpine and high latitude regions of Europe and western Asia and is sympatric with the recently described octoploid C. bithynica S.Jess., L.Lehm. & Bujnoch in north-central Turkey. Our analysis of a 6-region plastid DNA sequence dataset comprising 39 accessions of Cryptogramma R.Br., including 14 accessions of C. crispa and one accession of C. bithynica, revealed a deep genetic division between the accessions of C. crispa from western, northern, and central Europe and the accessions of C. crispa and C. bithynica from Turkey and the Caucasus Mountains. This legacy likely results from Pleistocene climate fluctuations and appears to represent incipient speciation between the eastern and western clades. These plastid DNA sequence data also demonstrate that the western clade of C. crispa, specifically the western Asian clade, is the maternal progenitor of C. bithynica. Our analysis of DNA sequence data from the biparentally inherited nuclear locus gapCp supports an autopolyploid origin of C. bithynica, with C. crispa as the sole progenitor. Key words: Cryptogramma, ferns, autopolyploidy, phylogeography, glacial refugium 1. -

<I>Equisetum Giganteum</I>
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 3-24-2009 Ecophysiology and Biomechanics of Equisetum Giganteum in South America Chad Eric Husby Florida International University, [email protected] DOI: 10.25148/etd.FI10022522 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Recommended Citation Husby, Chad Eric, "Ecophysiology and Biomechanics of Equisetum Giganteum in South America" (2009). FIU Electronic Theses and Dissertations. 200. https://digitalcommons.fiu.edu/etd/200 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida ECOPHYSIOLOGY AND BIOMECHANICS OF EQUISETUM GIGANTEUM IN SOUTH AMERICA A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in BIOLOGY by Chad Eric Husby 2009 To: Dean Kenneth Furton choose the name of dean of your college/school College of Arts and Sciences choose the name of your college/school This dissertation, written by Chad Eric Husby, and entitled Ecophysiology and Biomechanics of Equisetum Giganteum in South America, having been approved in respect to style and intellectual content, is referred to you for judgment. We have read this dissertation and recommend that it be approved. _______________________________________ Bradley C. Bennett _______________________________________ Jack B. Fisher _______________________________________ David W. Lee _______________________________________ Leonel Da Silveira Lobo O'Reilly Sternberg _______________________________________ Steven F. Oberbauer, Major Professor Date of Defense: March 24, 2009 The dissertation of Chad Eric Husby is approved. -

Monilophyte Mitochondrial Rps1 Genes Carry a Unique Group II Intron That Likely Originated from an Ancient Paralog in Rpl2
Downloaded from rnajournal.cshlp.org on September 26, 2021 - Published by Cold Spring Harbor Laboratory Press Monilophyte mitochondrial rps1 genes carry a unique group II intron that likely originated from an ancient paralog in rpl2 NILS KNIE, FELIX GREWE,1 and VOLKER KNOOP Abteilung Molekulare Evolution, IZMB–Institut für Zelluläre und Molekulare Botanik, Universität Bonn, D-53115 Bonn, Germany ABSTRACT Intron patterns in plant mitochondrial genomes differ significantly between the major land plant clades. We here report on a new, clade-specific group II intron in the rps1 gene of monilophytes (ferns). This intron, rps1i25g2, is strikingly similar to rpl2i846g2 previously identified in the mitochondrial rpl2 gene of seed plants, ferns, and the lycophyte Phlegmariurus squarrosus. Although mitochondrial ribosomal protein genes are frequently subject to endosymbiotic gene transfer among plants, we could retrieve the mitochondrial rps1 gene in a taxonomically wide sampling of 44 monilophyte taxa including basal lineages such as the Ophioglossales, Psilotales, and Marattiales with the only exception being the Equisetales (horsetails). Introns rps1i25g2 and rpl2i846g2 were likewise consistently present with only two exceptions: Intron rps1i25g2 is lost in the genus Ophioglossum and intron rpl2i846g2 is lost in Equisetum bogotense. Both intron sequences are moderately affected by RNA editing. The unprecedented primary and secondary structure similarity of rps1i25g2 and rpl2i846g2 suggests an ancient retrotransposition event copying rpl2i846g2 into rps1, for which we suggest a model. Our phylogenetic analysis adding the new rps1 locus to a previous data set is fully congruent with recent insights on monilophyte phylogeny and further supports a sister relationship of Gleicheniales and Hymenophyllales. Keywords: group II intron; RNA editing; intron transfer; reverse splicing; intron loss; monilophyte phylogeny INTRODUCTION and accordingly the genome sizes of most free-living bacteria, are an example for the latter (Ward et al. -

Horizontal Transfer of an Adaptive Chimeric Photoreceptor from Bryophytes to Ferns
Horizontal transfer of an adaptive chimeric photoreceptor from bryophytes to ferns Fay-Wei Lia,1, Juan Carlos Villarrealb, Steven Kellyc, Carl J. Rothfelsd, Michael Melkoniane, Eftychios Frangedakisc, Markus Ruhsamf, Erin M. Sigela, Joshua P. Derg,h, Jarmila Pittermanni, Dylan O. Burgej, Lisa Pokornyk, Anders Larssonl, Tao Chenm, Stina Weststrandl, Philip Thomasf, Eric Carpentern, Yong Zhango, Zhijian Tiano, Li Cheno, Zhixiang Yano, Ying Zhuo, Xiao Suno, Jun Wango, Dennis W. Stevensonp, Barbara J. Crandall-Stotlerq, A. Jonathan Shawa, Michael K. Deyholosn, Douglas E. Soltisr,s,t, Sean W. Grahamu, Michael D. Windhama, Jane A. Langdalec, Gane Ka-Shu Wongn,o,v,1, Sarah Mathewsw, and Kathleen M. Pryera aDepartment of Biology, Duke University, Durham, NC 27708; bSystematic Botany and Mycology, Department of Biology, University of Munich, 80638 Munich, Germany; cDepartment of Plant Sciences, University of Oxford, Oxford OX1 3RB, United Kingdom; dDepartment of Zoology, University of British Columbia, Vancouver, BC, Canada V6T 1Z4; eBotany Department, Cologne Biocenter, University of Cologne, 50674 Cologne, Germany; fRoyal Botanic Garden Edinburgh, Edinburgh EH3 5LR, Scotland; gDepartment of Biology and hHuck Institutes of the Life Sciences, Pennsylvania State University, University Park, PA 16802; iDepartment of Ecology and Evolutionary Biology, University of California, Santa Cruz, CA 95064; jCalifornia Academy of Sciences, San Francisco, CA 94118; kReal Jardín Botánico, 28014 Madrid, Spain; lSystematic Biology, Evolutionary Biology Centre, -
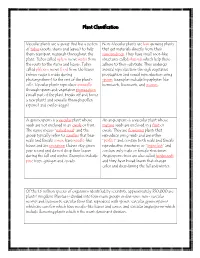
Plant Classification
Plant Classification Vascular plants are a group that has a system Non-Vascular plants are low growing plants of tubes (roots, stems and leaves) to help that get materials directly from their them transport materials throughout the surroundings. They have small root-like plant. Tubes called xylem move water from structures called rhizoids which help them the roots to the stems and leaves. Tubes adhere to their substrate. They undergo called phloem move food from the leaves asexual reproduction through vegetative (where sugar is made during propagation and sexual reproduction using photosynthesis) to the rest of the plant’s spores. Examples include bryophytes like cells. Vascular plants reproduce asexually hornworts, liverworts, and mosses. through spores and vegetative propagation (small part of the plant breaks off and forms a new plant) and sexually through pollen (sperm) and ovules (eggs). A gymnosperm is a vascular plant whose An angiosperm is a vascular plant whose seeds are not enclosed in an ovule or fruit. mature seeds are enclosed in a fruit or The name means “naked seed” and the ovule. They are flowering plants that group typically refers to conifers that bear reproduce using seeds and are either male and female cones, have needle-like “perfect” and contain both male and female leaves and are evergreen (leaves stay green reproductive structures or “imperfect” and year round and do not drop their leaves contain only male or female structures. during the fall and winter. Examples include Angiosperm trees are also called hardwoods pine trees, ginkgos and cycads. and they have broad leaves that change color and drop during the fall and winter. -

Guidance Document Pohakuloa Training Area Plant Guide
GUIDANCE DOCUMENT Recovery of Native Plant Communities and Ecological Processes Following Removal of Non-native, Invasive Ungulates from Pacific Island Forests Pohakuloa Training Area Plant Guide SERDP Project RC-2433 JULY 2018 Creighton Litton Rebecca Cole University of Hawaii at Manoa Distribution Statement A Page Intentionally Left Blank This report was prepared under contract to the Department of Defense Strategic Environmental Research and Development Program (SERDP). The publication of this report does not indicate endorsement by the Department of Defense, nor should the contents be construed as reflecting the official policy or position of the Department of Defense. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the Department of Defense. Page Intentionally Left Blank 47 Page Intentionally Left Blank 1. Ferns & Fern Allies Order: Polypodiales Family: Aspleniaceae (Spleenworts) Asplenium peruvianum var. insulare – fragile fern (Endangered) Delicate ENDEMIC plants usually growing in cracks or caves; largest pinnae usually <6mm long, tips blunt, uniform in shape, shallowly lobed, 2-5 lobes on acroscopic side. Fewer than 5 sori per pinna. Fronds with distal stipes, proximal rachises ocassionally proliferous . d b a Asplenium trichomanes subsp. densum – ‘oāli’i; maidenhair spleenwort Plants small, commonly growing in full sunlight. Rhizomes short, erect, retaining many dark brown, shiny old stipe bases.. Stipes wiry, dark brown – black, up to 10cm, shiny, glabrous, adaxial surface flat, with 2 greenish ridges on either side. Pinnae 15-45 pairs, almost sessile, alternate, ovate to round, basal pinnae smaller and more widely spaced. -

(Polypodiales) Plastomes Reveals Two Hypervariable Regions Maria D
Logacheva et al. BMC Plant Biology 2017, 17(Suppl 2):255 DOI 10.1186/s12870-017-1195-z RESEARCH Open Access Comparative analysis of inverted repeats of polypod fern (Polypodiales) plastomes reveals two hypervariable regions Maria D. Logacheva1, Anastasiya A. Krinitsina1, Maxim S. Belenikin1,2, Kamil Khafizov2,3, Evgenii A. Konorov1,4, Sergey V. Kuptsov1 and Anna S. Speranskaya1,3* From Belyaev Conference Novosibirsk, Russia. 07-10 August 2017 Abstract Background: Ferns are large and underexplored group of vascular plants (~ 11 thousands species). The genomic data available by now include low coverage nuclear genomes sequences and partial sequences of mitochondrial genomes for six species and several plastid genomes. Results: We characterized plastid genomes of three species of Dryopteris, which is one of the largest fern genera, using sequencing of chloroplast DNA enriched samples and performed comparative analysis with available plastomes of Polypodiales, the most species-rich group of ferns. We also sequenced the plastome of Adianthum hispidulum (Pteridaceae). Unexpectedly, we found high variability in the IR region, including duplication of rrn16 in D. blanfordii, complete loss of trnI-GAU in D. filix-mas, its pseudogenization due to the loss of an exon in D. blanfordii. Analysis of previously reported plastomes of Polypodiales demonstrated that Woodwardia unigemmata and Lepisorus clathratus have unusual insertions in the IR region. The sequence of these inserted regions has high similarity to several LSC fragments of ferns outside of Polypodiales and to spacer between tRNA-CGA and tRNA-TTT genes of mitochondrial genome of Asplenium nidus. We suggest that this reflects the ancient DNA transfer from mitochondrial to plastid genome occurred in a common ancestor of ferns. -

Molecular Phylogeny of Horsetails (Equisetum) Including Chloroplast Atpb Sequences
J Plant Res DOI 10.1007/s10265-007-0088-x SHORT COMMUNICATION Molecular phylogeny of horsetails (Equisetum) including chloroplast atpB sequences Jean-Michel Guillon Received: 9 November 2006 / Accepted: 21 March 2007 Ó The Botanical Society of Japan and Springer 2007 Abstract Equisetum is a genus of 15 extant species that dependent on vegetative reproduction for persistence and are the sole surviving representatives of the class Sphen- growth. The 15 species of Equisetum are grouped in two opsida. The generally accepted taxonomy of Equisetum subgenera based on morphological characters such as the recognizes two subgenera: Equisetum and Hippochaete. position of stomata: superficial in subgenus Equisetum (E. Two recent phylogenetical studies have independently arvense, E. bogotense, E. diffusum, E. fluviatile, E. pa- questioned the monophyly of subgenus Equisetum. Here, I lustre, E. pratense, E. sylvaticum, and E. telmateia), use original (atpB) and published (rbcL, trnL-trnF, rps4) sunken below the epidermal surface in subgenus Hippo- sequence data to investigate the phylogeny of the genus. chaete (E. giganteum, E. hyemale, E. laevigatum, Analyses of atpB sequences give an unusual topology, with E. myriochaetum, E. ramosissimum, E. scirpoides, and E. bogotense branching within Hippochaete. A Bayesian E. variegatum). A barrier seems to prevent hybridization analysis based on all available sequences yields a tree with between plants of the subgenera Equisetum and Hippo- increased resolution, favoring the sister relationships of chaete (Duckett 1979). E. bogotense with subgenus Hippochaete. Because characters found in the fossil record, such as large stems and persistent sheath teeth, are present in the Keywords Equisetum Á Evolution Á Horsetail Á Phylogeny sole E. -

Taxonomic, Phylogenetic, and Functional Diversity of Ferns at Three Differently Disturbed Sites in Longnan County, China
diversity Article Taxonomic, Phylogenetic, and Functional Diversity of Ferns at Three Differently Disturbed Sites in Longnan County, China Xiaohua Dai 1,2,* , Chunfa Chen 1, Zhongyang Li 1 and Xuexiong Wang 1 1 Leafminer Group, School of Life Sciences, Gannan Normal University, Ganzhou 341000, China; [email protected] (C.C.); [email protected] (Z.L.); [email protected] (X.W.) 2 National Navel-Orange Engineering Research Center, Ganzhou 341000, China * Correspondence: [email protected] or [email protected]; Tel.: +86-137-6398-8183 Received: 16 March 2020; Accepted: 30 March 2020; Published: 1 April 2020 Abstract: Human disturbances are greatly threatening to the biodiversity of vascular plants. Compared to seed plants, the diversity patterns of ferns have been poorly studied along disturbance gradients, including aspects of their taxonomic, phylogenetic, and functional diversity. Longnan County, a biodiversity hotspot in the subtropical zone in South China, was selected to obtain a more thorough picture of the fern–disturbance relationship, in particular, the taxonomic, phylogenetic, and functional diversity of ferns at different levels of disturbance. In 90 sample plots of 5 5 m2 along roadsides × at three sites, we recorded a total of 20 families, 50 genera, and 99 species of ferns, as well as 9759 individual ferns. The sample coverage curve indicated that the sampling effort was sufficient for biodiversity analysis. In general, the taxonomic, phylogenetic, and functional diversity measured by Hill numbers of order q = 0–3 indicated that the fern diversity in Longnan County was largely influenced by the level of human disturbance, which supports the ‘increasing disturbance hypothesis’. -

Fern Classification
16 Fern classification ALAN R. SMITH, KATHLEEN M. PRYER, ERIC SCHUETTPELZ, PETRA KORALL, HARALD SCHNEIDER, AND PAUL G. WOLF 16.1 Introduction and historical summary / Over the past 70 years, many fern classifications, nearly all based on morphology, most explicitly or implicitly phylogenetic, have been proposed. The most complete and commonly used classifications, some intended primar• ily as herbarium (filing) schemes, are summarized in Table 16.1, and include: Christensen (1938), Copeland (1947), Holttum (1947, 1949), Nayar (1970), Bierhorst (1971), Crabbe et al. (1975), Pichi Sermolli (1977), Ching (1978), Tryon and Tryon (1982), Kramer (in Kubitzki, 1990), Hennipman (1996), and Stevenson and Loconte (1996). Other classifications or trees implying relationships, some with a regional focus, include Bower (1926), Ching (1940), Dickason (1946), Wagner (1969), Tagawa and Iwatsuki (1972), Holttum (1973), and Mickel (1974). Tryon (1952) and Pichi Sermolli (1973) reviewed and reproduced many of these and still earlier classifica• tions, and Pichi Sermolli (1970, 1981, 1982, 1986) also summarized information on family names of ferns. Smith (1996) provided a summary and discussion of recent classifications. With the advent of cladistic methods and molecular sequencing techniques, there has been an increased interest in classifications reflecting evolutionary relationships. Phylogenetic studies robustly support a basal dichotomy within vascular plants, separating the lycophytes (less than 1 % of extant vascular plants) from the euphyllophytes (Figure 16.l; Raubeson and Jansen, 1992, Kenrick and Crane, 1997; Pryer et al., 2001a, 2004a, 2004b; Qiu et al., 2006). Living euphyl• lophytes, in turn, comprise two major clades: spermatophytes (seed plants), which are in excess of 260 000 species (Thorne, 2002; Scotland and Wortley, Biology and Evolution of Ferns and Lycopliytes, ed.