Download The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Medication Avoidance List for Skin Testing
D. L. Southern, M.D. A. Pedinoff, M.D. J. Caucino, D.O. H. Skolnick, M.D. K. Sikorski, M.D. S. Shah, M.D. N. Baman, M.D. Princeton 609-921-2202 Plainsboro 609-799-8111 Hamilton 609-888-1555 Flemington 908-782-0093 Fax #: 609-924-1468 Your skin testing or food/medication challenge has been scheduled for ______________________________________. The following is a list of commonly used antihistamines and medications, which can interfere with and reduce the accuracy of allergy testing. Please read carefully the times a medication needs to be discontinued prior to testing: Avoid the following antihistamine medications for 3 full days prior to skin testing: Benadryl=diphenydramine Phenergan cough syrup=promethazine Ru-Tuss, Triaminic=pheniramine Polaramine=tripelenamine Dramamine,Bonine=meclizine Bromfed, Dimetapp=brompheniramine Nolahist, Nolamine=phenindamine Actifed=triprolidine Trinalin=azatadine Avoid the following antihistamine medications for 5 full days prior to skin testing: Atarax=hydroxyzine Allegra=fexofenadine Polaramine=dexachlorpheniramine Zyrtec=cetirizine Avoid the following antihistamine medication for 7 full days prior to skin testing: Claritin=loratidine Clarinex=desloratidine Tavist=clemastine Xyzal=levocetirizine Avoid the following antihistamine for 9 full days prior to skin testing: Periactin=cyproheptadine Avoid the following nasal antihistamine sprays for 5 days prior to skin testing: Dymista, Astepro, Astelin=azelastine Patanase=olapatadine Avoid the following stomach medications for 3 days prior to skin testing: Zantac=ranitidine -

Customs Tariff - Schedule
CUSTOMS TARIFF - SCHEDULE 99 - i Chapter 99 SPECIAL CLASSIFICATION PROVISIONS - COMMERCIAL Notes. 1. The provisions of this Chapter are not subject to the rule of specificity in General Interpretative Rule 3 (a). 2. Goods which may be classified under the provisions of Chapter 99, if also eligible for classification under the provisions of Chapter 98, shall be classified in Chapter 98. 3. Goods may be classified under a tariff item in this Chapter and be entitled to the Most-Favoured-Nation Tariff or a preferential tariff rate of customs duty under this Chapter that applies to those goods according to the tariff treatment applicable to their country of origin only after classification under a tariff item in Chapters 1 to 97 has been determined and the conditions of any Chapter 99 provision and any applicable regulations or orders in relation thereto have been met. 4. The words and expressions used in this Chapter have the same meaning as in Chapters 1 to 97. Issued January 1, 2020 99 - 1 CUSTOMS TARIFF - SCHEDULE Tariff Unit of MFN Applicable SS Description of Goods Item Meas. Tariff Preferential Tariffs 9901.00.00 Articles and materials for use in the manufacture or repair of the Free CCCT, LDCT, GPT, UST, following to be employed in commercial fishing or the commercial MT, MUST, CIAT, CT, harvesting of marine plants: CRT, IT, NT, SLT, PT, COLT, JT, PAT, HNT, Artificial bait; KRT, CEUT, UAT, CPTPT: Free Carapace measures; Cordage, fishing lines (including marlines), rope and twine, of a circumference not exceeding 38 mm; Devices for keeping nets open; Fish hooks; Fishing nets and netting; Jiggers; Line floats; Lobster traps; Lures; Marker buoys of any material excluding wood; Net floats; Scallop drag nets; Spat collectors and collector holders; Swivels. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Prohibited Substances List
Prohibited Substances List This is the Equine Prohibited Substances List that was voted in at the FEI General Assembly in November 2009 alongside the new Equine Anti-Doping and Controlled Medication Regulations(EADCMR). Neither the List nor the EADCM Regulations are in current usage. Both come into effect on 1 January 2010. The current list of FEI prohibited substances remains in effect until 31 December 2009 and can be found at Annex II Vet Regs (11th edition) Changes in this List : Shaded row means that either removed or allowed at certain limits only SUBSTANCE ACTIVITY Banned Substances 1 Acebutolol Beta blocker 2 Acefylline Bronchodilator 3 Acemetacin NSAID 4 Acenocoumarol Anticoagulant 5 Acetanilid Analgesic/anti-pyretic 6 Acetohexamide Pancreatic stimulant 7 Acetominophen (Paracetamol) Analgesic/anti-pyretic 8 Acetophenazine Antipsychotic 9 Acetylmorphine Narcotic 10 Adinazolam Anxiolytic 11 Adiphenine Anti-spasmodic 12 Adrafinil Stimulant 13 Adrenaline Stimulant 14 Adrenochrome Haemostatic 15 Alclofenac NSAID 16 Alcuronium Muscle relaxant 17 Aldosterone Hormone 18 Alfentanil Narcotic 19 Allopurinol Xanthine oxidase inhibitor (anti-hyperuricaemia) 20 Almotriptan 5 HT agonist (anti-migraine) 21 Alphadolone acetate Neurosteriod 22 Alphaprodine Opiod analgesic 23 Alpidem Anxiolytic 24 Alprazolam Anxiolytic 25 Alprenolol Beta blocker 26 Althesin IV anaesthetic 27 Althiazide Diuretic 28 Altrenogest (in males and gelidngs) Oestrus suppression 29 Alverine Antispasmodic 30 Amantadine Dopaminergic 31 Ambenonium Cholinesterase inhibition 32 Ambucetamide Antispasmodic 33 Amethocaine Local anaesthetic 34 Amfepramone Stimulant 35 Amfetaminil Stimulant 36 Amidephrine Vasoconstrictor 37 Amiloride Diuretic 1 Prohibited Substances List This is the Equine Prohibited Substances List that was voted in at the FEI General Assembly in November 2009 alongside the new Equine Anti-Doping and Controlled Medication Regulations(EADCMR). -

Chapter 231. Poisons and Dangerous Substances Act 1952
Chapter 231. Poisons and Dangerous Substances Act 1952. Certified on: / /20 . INDEPENDENT STATE OF PAPUA NEW GUINEA. Chapter 231. Poisons and Dangerous Substances Act 1952. ARRANGEMENT OF SECTIONS. PART I – PRELIMINARY. 1. Interpretation. “automatic machine” “the British Pharmacopoeia” “container” “dangerous substance” “methylated spirit” “package” “poison” “poisons licence” “Poisons Register” “rectified spirit” “the regulations” “sell” “this Act” 2. Application. 3. Calculations of percentages. 4. Variation of schedules. PART II – LICENCES. 5. Poisons licence. 6. Revocation of poisons licences. PART III – SALE OF POISONS AND DANGEROUS SUBSTANCES. 7. Application of Part III to wholesalers. 8. Sale of things specified in Schedules 1 and 2. 9. Sale, etc., of things specified in Schedule 1. 10. Sale of things specified in Schedule 3. 11. Sale of things specified in Schedule 4. 12. Sale of things specified in Schedule 5. 13. Hawking poisons and dangerous substances. 14. Sale of poisons to persons under 18 years, etc. 15. Automatic vending machines. 16. Poisons Registers. 17. Entries in Poisons Registers. 18. Person unable to sign his name. 19. Sale on order by letter, etc. 20. Sale to medical practitioners, etc. 21. Application of Sections 14, 17, 18 and 19. 22. Records to be kept by medical practitioners, etc. PART IV – LABELLING AND PACKING POISONS AND DANGEROUS SUBSTANCES. 23. Sale of poisons and dangerous substances. 24. Sale of liquid dangerous substances. 25. Colouring of methylated spirits. 26. Sale of poisons. PART V – MISCELLANEOUS. 27. Drinking of methylated spirit prohibited. 28. Methylated spirit not to be sold for drinking purposes. 29. Inspection. 30. Offences in relation to the sale of poisons, etc. -

The Use of Stems in the Selection of International Nonproprietary Names (INN) for Pharmaceutical Substances
WHO/PSM/QSM/2006.3 The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances 2006 Programme on International Nonproprietary Names (INN) Quality Assurance and Safety: Medicines Medicines Policy and Standards The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 © World Health Organization 2006 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. -

Antihistamine Therapy in Allergic Rhinitis
CLINICAL REVIEW Antihistamine Therapy in Allergic Rhinitis Paul R. Tarnasky, MD, and Paul P. Van Arsdel, Jr, MD Seattle, Washington Allergic rhinitis is a common disorder that is associated with a high incidence of mor bidity and considerable costs. The symptoms of allergic rhinitis are primarily depen dent upon the tissue effects of histamine. Antihistamines are the mainstay of therapy for allergic rhinitis. Recently, a second generation of antihistamines has become available. These agents lack the adverse effect of sedation, which is commonly associated with older antihistamines. Current practice of antihistamine therapy in allergic rhinitis often involves random selection among the various agents. Based upon the available clinical trials, chlorpheniramine appears to be the most reasonable initial antihistaminic agent. A nonsedating antihis tamine should be used initially if a patient is involved in activities where drowsiness is dangerous. In this comprehensive review of allergic rhinitis and its treatment, the cur rent as well as future options in antihistamine pharmacotherapy are emphasized. J Fam Pract 1990; 30:71-80. llergic rhinitis is a common condition afflicting some defined by the period of exposure to those agents to which A where between 15 and 30 million people in the United a patient is sensitive. Allergens in seasonal allergic rhinitis States.1-3 The prevalence of disease among adolescents is consist of pollens from nonflowering plants such as trees, estimated to be 20% to 30%. Two thirds of the adult grasses, and weeds. These pollens generally create symp allergic rhinitis patients are under 30 years of age.4-6 Con toms in early spring, late spring through early summer, sequently, considerable costs are incurred in days lost and fall, respectively. -

Pre - PA Allowance Age 18 Years of Age Or Older Quantity 60 Grams Every 90 Days ______
DOXEPIN CREAM 5% (Prudoxin, Zonalon) Pre - PA Allowance Age 18 years of age or older Quantity 60 grams every 90 days _______________________________________________________________ Prior-Approval Requirements Age 18 years of age or older Diagnosis Patient must have the following: Moderate pruritus, due to atopic dermatitis (eczema) or lichen simplex chronicus AND the following: 1. Inadequate response, intolerance or contraindication to ONE medication in EACH of the following categories: a. Topical antihistamine (see Appendix I) b. High potency topical corticosteroid (see Appendix II) 2. Physician agrees to taper patient’s dose to the FDA recommended dose, and after tapered will only use for short-term pruritus relief (up to 8 days) a. Patients using over 60 grams of topical doxepin in 90 days be required to taper to 60 grams topical doxepin within 90 days Prior - Approval Limits Quantity 180 grams for 90 days Duration 3 months ___________________________________________________________________ Prior – Approval Renewal Requirements None (see appendix below) Doxepin 5% cream FEP Clinical Rationale DOXEPIN CREAM 5% (Prudoxin, Zonalon) APPENDIX I Drug Dosage Form Diphenhydramine Cream Phenyltoloxamine Lotion/ Cream Tripelennamine Cream Phendiamine Cream APPENDIX II Relative Potency of Selected Topical Corticosteroid Drug ProductsDosage Form Strength I. Very high potency Augmented betamethasone Ointment, Gel 0.05% dipropionate Clobetasol propionate Cream, Ointment 0.05% Diflorasone diacetate Ointment 0.05% Halobetasol propionate Cream, Ointment 0.05% II. High potency Amcinonide Cream, Lotion, 0.1% Augmented betamethasone Cream,Ointment Lotion 0.05% dipropionate Betamethasone Cream, Ointment 0.05% Betamethasonedipropionate valerate Ointment 0.1% Desoximetasone Cream, Ointment 0.25% Gel 0.05% Diflorasone diacetate Cream, Ointment 0.05% (emollient base) Fluocinonide Cream, Ointment, Gel 0.05% Halcinonide Cream, Ointment 0.1% Triamcinolone acetonide Cream, Ointment 0.5% III. -

San Diego ENT
San Diego ENT Allergy Skin Testing Instructions Make sure you review all of your medications with your doctor or the medical assistant when you are scheduled for your allergy test. DON’T’S: • Do note take over-the-counter antihistamines, cold medication, or cough syrup for 10 days prior to the test. This includes Benadryl, Claritin, Zyrtec, Allegra, loratadine, cetirizine, and fexofenadine, Tavist, Dramamine,Atarax, and others. • Do not take prescription antihistamines for 10 days prior to the test including Claritin, Zyrtec, Allegra, loratadine, cetirizine, fexofenadine, and Astelin nasal spray. Also stop antihistamine eye drops 10 days prior to testing. • Do not take beta blockers 5 days prior to testing. These include labetalol, metoprolol, carvedilol, and their brand name equivalents. • Do not take anti-acid medication for 48 hours prior to testing including Zantac, Pepcid, and Tagamet. You may continue to take proton pump inhibitors such as Prilosec, Nexium, Prevacid, Protonix, and Aciphex. • Do not take any sleeping medications for 48 hours prior to testing including Tylenol PM and Excedrin PM. DO’S: • Wear something comfortable that will allow access to your back or both upper arms on the day of testing. • You may continue to use nasal steroid sprays such as Flonase, Nasonex, Nasacort, and Rhinocort. Do not use Astelin. • Review the list of medications that need to be avoided below. Antihistamines to stop 10 days prior to testing: Generic Brand Name Acrivastine Semprex Azatadine Optimine, Trinalin Bropheniramine AccuHist, Bromfed, -
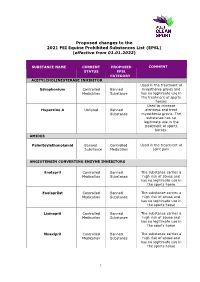
Proposed Changes to the 2021 FEI Equine Prohibited Substances List (EPSL) (Effective from 01.01.2022)
Proposed changes to the 2021 FEI Equine Prohibited Substances List (EPSL) (effective from 01.01.2022) SUBSTANCE NAME CURRENT PROPOSED COMMENT STATUS EPSL CATEGORY ACETYLCHOLINESTERASE INHIBITOR Used in the treatment of Edrophonium Controlled Banned myasthenia gravis and Medication Substance has no legitimate use in the treatment of sports horses Used to increase Huperzine A Unlisted Banned alertness and treat Substance myasthenia gravis. The substance has no legitimate use in the treatment of sports horses. AMIDES Palmitoylethanolamid Banned Controlled Used in the treatment of Substance Medication joint pain ANGIOTENSIN CONVERTING ENZYME INHIBITORS Enalapril Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse Enalaprilat Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse Lisinopril Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse Moexipril Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse 1 Perindoprilat Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse ANTIHISTAMINES Antazoline Controlled Banned The substance has no Medication Substance legitimate use in the sports horse Azatadine Controlled Banned The substance has Medication Substance sedative effects -
![With [3H]Mepyramine (Trieyclic Antidepressants/Antihistamine/Neurotransmitter/Amitriptyline) VINH TAN TRAN, RAYMOND S](https://docslib.b-cdn.net/cover/2862/with-3h-mepyramine-trieyclic-antidepressants-antihistamine-neurotransmitter-amitriptyline-vinh-tan-tran-raymond-s-1512862.webp)
With [3H]Mepyramine (Trieyclic Antidepressants/Antihistamine/Neurotransmitter/Amitriptyline) VINH TAN TRAN, RAYMOND S
Proc. Nati. Acad. Sci. USA Vol. 75, No. 12, pp. 6290-6294,, December 1978 Neurobiology Histamine H1 receptors identified in mammalian brain membranes with [3H]mepyramine (trieyclic antidepressants/antihistamine/neurotransmitter/amitriptyline) VINH TAN TRAN, RAYMOND S. L. CHANG, AND SOLOMON H. SNYDER* Departments of Pharmacology and Experimental Therapeutics, and Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205 Communicated by Julius Axelrod, August 30,1978 ABSTRACT The antihistamine [3H mepyramine binds to Male Sprague-Dawley rats (150-200 g) were killed by cer- HI histamine receptors in mammalian brain membranes. vical dislocation, their brains were rapidly removed and ho- Potencies of H1 antihistamines at the binding sites correlate mogenized with a Polytron for 30 min (setting 5) in 30 vol of with their pharmacological antihistamine effects in the guinea pig ileum. Specific [3Himepyramine binding is saturable with ice-cold Na/K phosphate buffer (50 mM, pH 7.5), and the a dissociation constant of about 4 nM in both equilibrium and suspension was centrifuged (50,000 X g for 10 min). The pellet kinetic experiments and a density of 10pmolper gram ofwhole was resuspended in the same volume of fresh buffer and cen- brain. Some tricyclic antidepressants are potent inhibitors of trifuged, and the final pellet was resuspended in the original secific [3Hmepamine binding. Regional variations of volume of ice-cold buffer by Polytron homogenization. Calf [3Hjmepyramine ing do not correlate with variations in brains were obtained from a local abattoir within 2 hr after the endogeneous histamine and histidine decarboxylase activity. death of the animals and transferred to the laboratory in ice- Histamine is a neurotransmitter candidate in mammalian brain cold saline. -

Allergy Testing Instructions
ALLERGY TESTING INSTRUCTIONS Antihistamines, prescription and over the counter ones, negatively affect the results of skin tests. These medications and instructions are outlined below. In addition, many over the counter cold and cough medications, acid reducers / medications and eye drops contain medications that act as antihistamines and need to be stopped before skin testing. If you are not sure medications, please call our office. If these medications are not stopped the required number of days before the appointment, you will not be able to complete the skin testing on the day of appointment. The testing may have to be rescheduled or other options may be considered. Stop these medications 14 days before your appointment: Stop these oral antihistamines 4-5 days before your 1. Atarax®, Vistaril®, Marax (Hydroxyzine) appointment: 1. Alavert® (Loratadine) 2. Allegra® (Fexofenadine) 3. Clarinex® (Desloratadine) 4. Claritin® (Loratadine) 5. Loratadine (Claritin, Alavert) Stop these oral antihistamines 4-5 days before your appointment: Actifed Meclizine (Antivert)Methdilazine HCI (Tacaryl) Naldecone Antihist Novafed-A Ornade Patanase Astelin®, Astepro (Azelastine) Axid® (nizatidine) Pepcid® (famotidine) Phenergan (Promethazine) Azatadine (Optimine, Trinalin) Benadryl (Diphenhydramine) Phenindamine (Nolamine, Nolahist) Bromfed Pheniramine (Polyhistine D) Brompheniramine Poly-Histine-D Cabinoxamine (Rondec) Chlopheniramine (Chlortrimeton) Promethazine HCI (Phenegan) Pyrilamine (Kronohist, Clemastine (Tavist) Cyproheptadine (Periactin) Deconamine Rynatan) Rynatan Dimenhydrinate (Dramamine) Dimetapp Tagamet® (cimetidine) Tavist Diphenhydramine (Benadryl) Diphenylpyraline (Hispril) Trimeprazine (Temaril) Trinalin Doxylamine (Bendectin, Nyquil) Drixoral Triprolidine (Actifed) Dura-tab Valium (Lorazepam, Ativan) ie. Sleeping Pills Flexeril- ie. Muscle Relaxants, etc. Kronofed Xyzal (Levocetirizine) Zantac® (ranitidine) Zyrtec® (Cetirizine) If you are taking an oral/nasal antihistamine that is not listed above, stop the medicine for 4 days before your appointment.