Conservation Advice Argyrotegium Nitidulum Shining Cudweed
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Vascular Plants of Massachusetts
The Vascular Plants of Massachusetts: The Vascular Plants of Massachusetts: A County Checklist • First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Somers Bruce Sorrie and Paul Connolly, Bryan Cullina, Melissa Dow Revision • First A County Checklist Plants of Massachusetts: Vascular The A County Checklist First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Massachusetts Natural Heritage & Endangered Species Program Massachusetts Division of Fisheries and Wildlife Natural Heritage & Endangered Species Program The Natural Heritage & Endangered Species Program (NHESP), part of the Massachusetts Division of Fisheries and Wildlife, is one of the programs forming the Natural Heritage network. NHESP is responsible for the conservation and protection of hundreds of species that are not hunted, fished, trapped, or commercially harvested in the state. The Program's highest priority is protecting the 176 species of vertebrate and invertebrate animals and 259 species of native plants that are officially listed as Endangered, Threatened or of Special Concern in Massachusetts. Endangered species conservation in Massachusetts depends on you! A major source of funding for the protection of rare and endangered species comes from voluntary donations on state income tax forms. Contributions go to the Natural Heritage & Endangered Species Fund, which provides a portion of the operating budget for the Natural Heritage & Endangered Species Program. NHESP protects rare species through biological inventory, -

News from the Serious Recognition of the Australian Alps’ Worth Is Coming in Thick and Fast
#37 2008 news from the Serious recognition of the Australian Alps’ worth is coming in thick and fast. Most of us who live and work in and around the Alps have appreci- ated this landscape’s priceless value for many years, but 2008 will be remembered as the year that this iconic Australian landscape was given not just one, but two significant pats on the back. And in the big picture, these two ‘pats’ are more like two good whacks because they both carry serious clout. ALPS GETS NATIONAL HERITAGE LISTING The first came in June when the Alps region was declared one of Australia’s iconic destinations under the banner of the National Landscapes conservation/tourism initiative. The Australian Alps joins other iconic landscapes such as Kakadu and the Red Centre as a destination to appeal to international experience seekers. And being part of the initiative has brought with it all the resources on offer – especially support for the Alps as it works to develop its own strategic tourism plan as part of the initiative. And then came the second lot of big news. In early November the Federal Minister for the Environ- ment, Heritage and the Arts, Peter Garrett, announced National Heritage Listing* for the Australian Alps National Parks, an area of over 1.6 million hectares spanning Victoria, NSW and the ACT. Said Peter Garrett, “the listing of the Australian Alps National Parks recognises the outstanding natural, Indigenous and historic values of this iconic landscape.” Peter Jacobs, Convenor of the Australian Alps Liaison Committee explains. “The National Heritage Listing gives the Alps national significance. -

Argyrotegium Fordianum
Argyrotegium fordianum FAMILY: ASTERACEAE BOTANICAL NAME: Argyrotegium fordianum, (M.Gray) J.M.Ward & Breitw., N.Z. J. Bot. 41: 609 (2003) COMMON NAME: soft cottonleaf COMMONWEALTH STATUS: (EPBC Act) Not Listed TASMANIAN STATUS: (TSP Act) rare Argyrotegium fordianum. Tasmanian Herbarium specimen. Description An underground stem forming (rhizomatous), tufted perennial forb that is sometimes mat-like. Leaves: The leaves arise mostly from the base of the plant and are crowded towards the branch-tips. They are between 1.5-4 cm long and 5-10 mm wide. Both leaf surfaces are silvery-grey with dense cottony hairs. Flowers: The flowering stems are erect or sloping upward and between 4-9 cm tall (up to 15 cm in fruit) with 4-10 leaves only slightly smaller than the ones at the base of the plant. The flowerheads are arranged in clusters as the ends of the flowering stems. Each flowerhead contains between 5-15 florets. Flowering is from December to February (Flora of Victoria). Fruit: The fruit is small, dry, leathery and hairless, between 1.3-1.5 mm long (description from Walsh & Entwhisle 1999). This species was previously known as Euchiton fordianus or Gnaphalium fordianum. Ecology and Management There is currently no information available regarding the ecology and management of this species. Conservation Status Assessment Reassessment of Argyrotegium fordianum may be warranted due to the paucity of records though more information is required for a meaningful reassessment. Further Information Walsh, NG & Entwhisle, TJ eds 1999, Flora of Victoria, Volume 4, Inkata Press, Melbourne. Threatened Species Section – Department of Primary Industries, Parks, Water and Environment Argyrotegium fordianum Preceding text last modified 18/7/2005. -

ABSTRACTS 117 Systematics Section, BSA / ASPT / IOPB
Systematics Section, BSA / ASPT / IOPB 466 HARDY, CHRISTOPHER R.1,2*, JERROLD I DAVIS1, breeding system. This effectively reproductively isolates the species. ROBERT B. FADEN3, AND DENNIS W. STEVENSON1,2 Previous studies have provided extensive genetic, phylogenetic and 1Bailey Hortorium, Cornell University, Ithaca, NY 14853; 2New York natural selection data which allow for a rare opportunity to now Botanical Garden, Bronx, NY 10458; 3Dept. of Botany, National study and interpret ontogenetic changes as sources of evolutionary Museum of Natural History, Smithsonian Institution, Washington, novelties in floral form. Three populations of M. cardinalis and four DC 20560 populations of M. lewisii (representing both described races) were studied from initiation of floral apex to anthesis using SEM and light Phylogenetics of Cochliostema, Geogenanthus, and microscopy. Allometric analyses were conducted on data derived an undescribed genus (Commelinaceae) using from floral organs. Sympatric populations of the species from morphology and DNA sequence data from 26S, 5S- Yosemite National Park were compared. Calyces of M. lewisii initi- NTS, rbcL, and trnL-F loci ate later than those of M. cardinalis relative to the inner whorls, and sepals are taller and more acute. Relative times of initiation of phylogenetic study was conducted on a group of three small petals, sepals and pistil are similar in both species. Petal shapes dif- genera of neotropical Commelinaceae that exhibit a variety fer between species throughout development. Corolla aperture of unusual floral morphologies and habits. Morphological A shape becomes dorso-ventrally narrow during development of M. characters and DNA sequence data from plastid (rbcL, trnL-F) and lewisii, and laterally narrow in M. -

Wagner-Etal97.Pdf
Records of the Hawaii Biological Survey for 1996. Bishop 51 Museum Occasional Papers 48: 51-65. (1997) Contributions to the Flora of the Hawai‘i. VI1 WARREN L. WAGNER 2, ROBYNN K. SHANNON (Department of Botany, MRC 166, National Museum of Natural History, Smithsonian Institution, Washington DC 20560, USA), and DERRAL R. HERBST3 (U.S. Army Corps of Engineers, CEPOD-ED-ES, Fort Shafter, HI 96858, USA) As discussed in previous papers published in the Records of the Hawaii Biological Survey (Wagner & Herbst, 1995; Lorence et al., 1995; Herbst & Wagner, 1996; Shannon & Wagner, 1996), recent collecting efforts, continued curation of collections at Bishop Museum and the National Museum of Natural History (including processing of backlogs), and review of relevant literature all continue to add to our knowledge about the Hawaiian flora. When published, this information supplements and updates the treatments in the Manual of the flowering plants of Hawai‘i (Wagner et al., 1990). In just the last 2 years, in the Records of the Hawaii Biological Survey alone, new records for 265 taxa of flow- ering plants have been reported from the Hawaiian Islands. In this paper we report an additional 13 new state records (from Kaua‘i, O‘ahu, Moloka‘i, and Hawai‘i), 14 new island records (from Midway Atoll, Kaua‘i, O‘ahu, Maui, Lana‘i, Kaho‘olawe, and Hawai‘i), 8 taxonomic changes, and 2 corrections of identification. All specimens are determined by the authors except as indicated. Amaranthaceae Amaranthus retroflexus L. New state record This coarse, villous, monoecious herb 5–30 dm tall apparently was naturalized in the Hawaiian Islands 20 years ago, but its current status is not known. -

2016 Census of the Vascular Plants of Tasmania
A CENSUS OF THE VASCULAR PLANTS OF TASMANIA, INCLUDING MACQUARIE ISLAND MF de Salas & ML Baker 2016 edition Tasmanian Herbarium, Tasmanian Museum and Art Gallery Department of State Growth Tasmanian Vascular Plant Census 2016 A Census of the Vascular Plants of Tasmania, Including Macquarie Island. 2016 edition MF de Salas and ML Baker Postal address: Street address: Tasmanian Herbarium College Road PO Box 5058 Sandy Bay, Tasmania 7005 UTAS LPO Australia Sandy Bay, Tasmania 7005 Australia © Tasmanian Herbarium, Tasmanian Museum and Art Gallery Published by the Tasmanian Herbarium, Tasmanian Museum and Art Gallery GPO Box 1164 Hobart, Tasmania 7001 Australia www.tmag.tas.gov.au Cite as: de Salas, M.F. and Baker, M.L. (2016) A Census of the Vascular Plants of Tasmania, Including Macquarie Island. (Tasmanian Herbarium, Tasmanian Museum and Art Gallery. Hobart) www.tmag.tas.gov.au ISBN 978-1-921599-83-5 (PDF) 2 Tasmanian Vascular Plant Census 2016 Introduction The classification systems used in this Census largely follow Cronquist (1981) for flowering plants (Angiosperms) and McCarthy (1998) for conifers, ferns and their allies. The same classification systems are used to arrange the botanical collections of the Tasmanian Herbarium and by the Flora of Australia series published by the Australian Biological Resources Study (ABRS). For a more up-to-date classification of the flora refer to The Flora of Tasmania Online (Duretto 2009+) which currently follows APG II (2003). This census also serves as an index to The Student’s Flora of Tasmania (Curtis 1963, 1967, 1979; Curtis & Morris 1975, 1994). Species accounts can be found in The Student’s Flora of Tasmania by referring to the volume and page number reference that is given in the rightmost column (e.g. -

Typification of Gnaphalium Collinum Var. Monocephalum (Gnaphalieae
C.Nuytsia Flann, 20: P.G. 1–5 Wilson (2010) & J.J. Wieringa, Typification of Gnaphalium collinum 1 Typification ofGnaphalium collinum var. monocephalum (Gnaphalieae: Asteraceae) and clarification of related material Christina Flann1, Paul G. Wilson2 and Jan J. Wieringa1 1Netherlands Centre for Biodiversity Naturalis (section NHN), Wageningen University Branch, Biosystematics Group, Generaal Foulkesweg 37, 6703BL Wageningen, The Netherlands 2Western Australian Herbarium, Department of Environment and Conservation, Locked Bag 104, Bentley Delivery Centre, Bentley, Western Australia 6983 Email: [email protected] Abstract Flann, C., Wilson, P.G. & Wieringa, J.J. Typification ofGnaphalium collinum var. monocephalum (Gnaphalieae: Asteraceae) and clarification of related material.Nuytsia 20: 1–5 (2010). The protologue of Gnaphalium collinum var. monocephalum Hook.f. cites three gatherings which are now considered to be referable to three different taxa known by the names Euchiton lateralis (C.J.Webb) Breitw. & J.M.Ward, Euchiton traversii (Hook.f.) Holub and Argyrotegium mackayi (Buchanan) J.M.Ward & Breitw. This has caused confusion regarding the typification and application of J.D.Hooker’s varietal name. This article resolves the uncertainty and provides a corrected synonymy for all the taxa involved. Introduction Recent taxonomic work in the genus Euchiton Cass. (Flann et al. 2008) has raised questions about the name Gnaphalium collinum var. monocephalum Hook.f. (1859) as there has been confusion regarding its application resulting from issues of typification (Drury 1972, Ward et al. 2003). The protologue included three gatherings which are now referred to three different taxa known as Euchiton lateralis (C.J.Webb) Breitw. & J.M.Ward, Euchiton traversii (Hook.f.) Holub and Argyrotegium mackayi (Buchanan) J.M.Ward & Breitw. -

Gamochaeta Pensylvanica (Asteraceae) Is Established in the New York Flora
Atha, D., R. Alvarez, D. Feeser, M. Feder, Z. Wang, and R. Kelly. 2016. Gamochaeta pensylvanica (Asteraceae) is established in the New York flora. Phytoneuron 2016-22: 1–4. Published 3 March 2016. ISSN 2153 733X GAMOCHAETA PENSYLVANICA (ASTERACEAE) IS ESTABLISHED IN THE NEW YORK FLORA DANIEL ATHA Center for Conservation Strategy New York Botanical Garden 2900 Southern Blvd. Bronx, New York 10458 Author for correspondence: [email protected] REGINA ALVAREZ Queensborough Community College 222-05 56th Ave. Bayside, New York 11364 DAN FEESER Institute for Urban Parks, Central Park Conservancy 14 East 60th St. New York, New York 10022 MICHAEL FEDER New York City Department of Parks and Recreation 85-20 66th Rd. Rego Park, New York 11374 ZIHAO WANG Center for Conservation Strategy, New York Botanical Garden 2900 Southern Blvd. Bronx, New York 10458 RICH KELLY Long Island Botanical Society 622 South 8th St. New Hyde Park, New York 11040 ABSTRACT Recent field work and study of herbarium specimens document Gamochaeta pensylvanica as an established element in the spontaneous flora of New York State. The species was first collected in New York in 1938, but the sole specimen was misidentified as Gamochaeta purpurea . Gamochaeta pensylvanica is reported here with specimens from Bronx, Kings, and Queens counties and observations from Nassau County. A key based on living and preserved specimens distinguishing G. purpurea from G. pensylvanica is provided. Of the 12 species of Gamochaeta recognized in North America (Nesom, 2006), only G. purpurea (L.) Cabrera has previously been reported for New York state (Nesom 2006; Weldy et al. 2015), where it has been considered rare (S3) (Weldy et al. -

Argyrotegium Nitidulum Nitidulum (Shining Cottonleaf)
ArgyrotegiumNotesheet for Argyrotegium nitidulum nitidulum (shining cottonleaf) shining cottonleaf T A S M A N I A N T H R E A T E N E D S P E C I E S N O T E S H E E T Image courtesy of Tasmanian Herbarium Scientific name: Argyrotegium nitidulum (Hook.f.) J.M.Ward & Breitw., New Zealand J. Bot. 41: 609 (2003) Common name: shining cottonleaf (Wapstra et al. 2005) Group: vascular plant, dicotyledon, family Asteraceae Status: Threatened Species Protection Act 1995: vulnerable Environment Protection and Biodiversity Conservation Act 1999: Vulnerable Distribution: Endemic status: not endemic to Tasmania Tasmanian NRM regions: North Figure 1. Distribution of Argyrotegium nitidulum in Plate 1. Argyrotegium nitidulum Tasmania, showing IBRA bioregions (image courtesy of Tasmanian Herbarium) 1 Threatened Species Section – Department of Primary Industries, Parks, Water and Environment Notesheet for Argyrotegium nitidulum (shining cottonleaf) SUMMARY: Argyrotegium nitidulum (shining elongate to 3 cm as the fruits develop. The cottonleaf) is a mat- or cushion-forming 7 to 9 mm bracts on the inner edge of the perennial daisy that colonises damp gaps and flower heads are oblong and brown with long bare ground in alpine or subalpine areas. Only hairs at the base, and are glabrous and shiny recognised as occurring in Tasmania in 2003, with a thin dry texture above. The achenes the species is not well known in the State, (single seeded fruits) are smooth, glabrous, where it has only been confirmed from Ben obovate and 1.3 to 1.5 mm long with pappus Lomond in the north east. -
Supporting Information
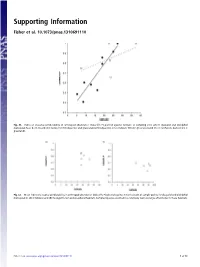
Supporting Information Fisher et al. 10.1073/pnas.1310691110 Fig. S1. Index of seasonal predictability in arthropod abundance (Colwell’s P) plotted against latitude of sampling sites where dasyurid and didelphid marsupials have been recorded in rainforest (filled points) and grassland (unfilled points). Lines indicate fitted regressions (solid line = rainforest, dashed line = grassland). Fig. S2. Mean index of seasonal predictability in arthropod abundance (Colwell’s P) plotted against mean latitude of sample points for dasyurid and didelphid marsupials in (A) shrubland and (B) Eucalypt forest and woodland habitats. Sampled species occurred in a relatively narrow range of latitudes in these habitats. Fisher et al. www.pnas.org/cgi/content/short/1310691110 1of13 Fig. S3. Phylogeny of insectivorous marsupials with known life history data, based on ref. 1 with updates from ref. 2. 1. Cardillo M, Bininda-Emonds ORP, Boakes E, Purvis A (2004) A species-level phylogenetic supertree of marsupials. J Zool 264(1):11–31. 2. Fritz SA, Bininda-Emonds ORP, Purvis A (2009) Geographical variation in predictors of mammalian extinction risk: Big is bad, but only in the tropics. Ecol Lett 12(6):538–549. Fisher et al. www.pnas.org/cgi/content/short/1310691110 2of13 Fisher et al. Table S1. Reproductive traits and diets of insectivorous marsupials Latitude (south) www.pnas.org/cgi/content/short/1310691110 Proportion Proportion Female for Habitat of of age species class for females males Male Breeding Copulation Litters Scrotal at first with species Genus species -

Kosciuszko National Park Fire Management Strategydownload
Kosciuszko National Park Fire Management Strategy 2008–2013 Acknowledgment The Kosciuszko National Park Fire Management Strategy 2008 - 2013 recognises that the Park is within landscape that gives identity to Aboriginal people, who have traditional and historical connections to this land. Aboriginal people are recognised and respected as the original custodians of the lands, waters, animals and plants now within the Park. Their living and spiritual connections with the land through traditional laws, customs and beliefs passed on from their ancestors are also recognised. NPWS will continue to be committed to actively engage traditional custodians and relevant Aboriginal organisations, in protecting managing and interpreting the needs of Kosciuszko National Park. The landscapes of Kosciuszko National Park are the sum total of the interactions between the basic natural elements of water, air, rocks, soils, fire, plants and animals. Each of these living and non-living attributes have been altered by thousands of years of human habitation and use which have left behind layers of human artefacts, memories, stories and meanings. The management of these attributes, and the values bestowed upon them, is a complex task and will not always be based upon a complete understanding of the implications of individual management decisions. A precautionary and adaptive approach to management is required - one that appreciates the interconnective and inseparable nature of the elements of the landscape. (NPWS, 2006c pg 37) Kosciusko National Park Fire Management Strategy 2008 - 2013 ACKNOWLEDGMENTS This Fire Management Strategy was prepared by Jamie Molloy with the assistance of staff from the Snowy Mountains and South West Slopes Regions of the National Parks and Wildlife Service, staff from the Threatened Species Unit, the Reserve Conservation Planning & Performance Unit, and the Public Affairs Division of the Department of Environment and Climate Change. -

Vale of Belvoir Reserve Supplement Contents Key
BUSH BLITZ SPECIES DISCOVERY PrOGRAM Vale of Belvoir Reserve Supplement Contents Key Appendix A: Species Lists 3 ¤ = Previously recorded on the reserve and Fauna 4 found on this survey Vertebrates 4 * = New record for this reserve Birds 4 ^ = Exotic/Pest Fishes 5 # = EPBC listed Frogs 5 ~ = TSP listed Putative new species Mammals 6 Previously recorded on the reserve but not found on Reptiles 6 this survey Invertebrates 7 Bees 7 EPBC = Environment Protection and Biodiversity Butterflies and Moths 7 Conservation Act 1999 (Commonwealth) Flies 7 TSP = Threatened Species Protection Act 1995 Beetles 7 (Tasmania) True Bugs 8 Grasshoppers 8 Dragonflies 8 Caddisflies 9 Millipedes 9 Spiders 10 Crustaceans 10 Snails and Slugs 11 Flora 12 Flowering Plants 12 Gymnosperms 13 Ferns and Fern Allies 13 Liverworts 14 Mosses 14 Fungi 15 Appendix B: Rare and Threatened Species 17 Appendix C: Exotic and Pest Species 19 2 Bush Blitz survey report — Tasmania 2010 Appendix A: Species Lists Nomenclature and taxonomy used in this appendix are consistent with that from the Australian Faunal Directory (AFD), the Australian Plant Name Index (APNI) and the Australian Plant Census (APC). Current at April 2012 Vale of Belvoir Reserve Supplement 3 Fauna Vertebrates Birds Family Species Common name Acanthizidae Acanthiza ewingii Tasmanian Thornbill Acanthiza pusilla * Brown Thornbill Acanthornis magna Scrubtit Calamanthus fuliginosus * Striated Fieldwren Sericornis frontalis * White-browed Scrubwren Sericornis humilis * Tasmanian Scrubwren Accipitridae Aquila audax *